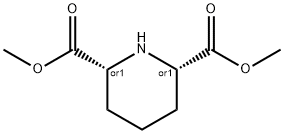
cis-Piperidine-2,6-dicarboxylic acid dimethyl ester synthesis
- Product Name:cis-Piperidine-2,6-dicarboxylic acid dimethyl ester
- CAS Number:59234-46-7
- Molecular formula:C9H15NO4
- Molecular Weight:201.22
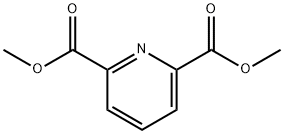
5453-67-8
294 suppliers
$6.00/5g

59234-46-7
11 suppliers
inquiry
Yield:59234-46-7 97%
Reaction Conditions:
with 20% Pd/C;hydrogen in methanol at 50; under 45004.5 Torr;
Steps:
cis-O1-tert-butyl O2,O6-dimethyl piperidine-1,2,6-tricarboxylate 5
A solution of dimethyl pyridine-2,6-dicarboxylate (2.00 g, 10.2 mmol) in methanol (400 mL) was passed through the H-Cube flow hydrogenator at a flow rate of 1 mL/min, over a 20% Pd on carbon catalyst bed, at 50 oC under 60 bar of hydrogen pressure. The reaction was complete after a single run, the solvent was then removed at reduced pressure to afford cis-dimethyl-piperidine-2,6-dicarboxylate (2.01 g, 97%) as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 3.64 (s, 6H), 3.35 (dd, J=2.0, 11.4 Hz, 2H), 2.33 (s, 1H), 1.89 - 1.78 (m, 3H), 1.53 - 1.38 (m, 1H), 1.29 - 1.15 (m, 2H). 13C NMR (100 MHz, DMSO-d6) δ 172.8, 57.5, 51.6, 28.3, 23.5. ESI-HRMS: calculated for C9H15NO4 [(M+H)+]: 202.1001. Found: 202.1074. To a solution of cis-dimethyl-piperidine-2,6-dicarboxylate (4.00 g, 19.9 mmol) in toluene (150 ml) was added di-tert-butyl dicarbonate (6.51 g, 29.8 mmol). Then the mixture was heated at 95 oC for 16h. The solvent was then removed at reduced pressure. The residue was purified by flash column chromatography eluting with a gradient of EA:PE (10:100 to 30:100) to afford compound (±)-5 (5.80 g, 97%) as a colorless oil. 1H NMR (400 MHz, CD3OD) δ: 4.82 - 4.74 (m, 2H), 3.70 (s, 6H), 2.19 - 2.09 (m, 2H), 1.77 - 1.63 (m, 3H), 1.54 (m, 1H), 1.49 (s, 9H).
References:
Wu, Guolong;Wang, Di;Zhu, Wei;Shen, Hong;Liu, Haixia;Fu, Lei [Synthetic Communications,2019,vol. 49,# 1,p. 12 - 21] Location in patent:supporting information
499-83-2
640 suppliers
$6.00/10g

59234-46-7
11 suppliers
inquiry
138-60-3
269 suppliers
$6.00/5g

59234-46-7
11 suppliers
inquiry