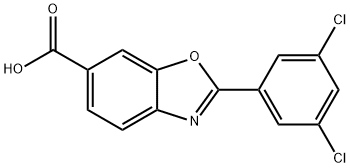
2-(3,5-Dichlorophenyl)-6-benzoxazole carboxylic acid synthesis
- Product Name:2-(3,5-Dichlorophenyl)-6-benzoxazole carboxylic acid
- CAS Number:594839-88-0
- Molecular formula:C14H7Cl2NO3
- Molecular Weight:308.12

Simoes, Carlos J. V.; Almeida, Zaida L.; Costa, Dora; Jesus, Catarina S. H.; Cardoso, Ana L.; Almeida, Maria R.; Saraiva, Maria J.; Pinho e Melo, Teresa M. V. D.; Brito, Rui M. M.
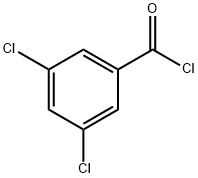
2905-62-6
369 suppliers
$13.43/1gm:
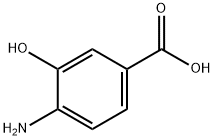
2374-03-0
350 suppliers
$12.72/1gm:

594839-88-0
153 suppliers
$59.00/5mg
Yield:594839-88-0 113 g
Reaction Conditions:
Stage #1: 3,5-dichlorobenzoyl chloride;4-aminosalicylic acid in 1-methyl-pyrrolidin-2-one;toluene at 26 - 120; for 6 h;
Stage #2: with toluene-4-sulfonic acid in 1-methyl-pyrrolidin-2-one;toluene at 120 - 145; for 21 h;Temperature;
Steps:
1; 2 Example-2: Preparation of Tafamidis
To a mixture of 4-amino 3-hydroxybenzoic acid (75 g) and 25% N-methyl 2- pyrrolidone in toluene (750 mL), 3,5-dichlorobenzoyl chloride (112.9 g) was added at 26 °C. The reaction mixture was heated to 120 °C and stirred at the same temperature for 6 hours. After the completion of reaction, p- toluenesulfonic acid monohydrate (46.6 g) was added slowly to the reaction mixture at 120 °C. The reaction mixture was heated further to 145 °C and stirred for 16 hours at the same temperature. Further, p-toluenesulfonic acid monohydrate (18.63 g) was added slowly and stirred for 5 hours at the same temperature. The mixture was cooled to 26 °C and added to water (4.5 litres). The reaction mixture was stirred at 26 °C for 16 hours. The solids were allowed to settle down and the supernatant liquid was separated out. Water (4.5 litres) was added to the solids and the suspension was stirred at 26 °C for 5 hours and filtered. The solid was dried in a hot air oven at 80 °C. The dried compound was stirred with 30% ethyl acetate in hexane (0.75 litres) for 2 hours at 26 °C and filtered. The solid was washed with 30% ethyl acetate in hexane (2 x 0.375 litres) and dried under vacuum for 2 hours to obtain a pale brown colored solid with HPLC purity of 93.38%. The dry solid (129 g) was combined with tetrahydrofuran (3.87 litres) and the reaction mixture was heated to 60 °C. Activated carbon (12.9 g) was added to the mixture at 60 °C and the suspension was stirred for 1 hour at the same temperature. The mixture was then filtered through celite bed. The filtrate was cooled to 25 °C and added slowly to water (5.16 litres), and the suspension was stirred for 2 hours at 25 °C. The solid was filtered and washed with water (1.29 litres), followed by hexanes (2 x 1.29 litres). The solid was dried under vacuum for 4 hours to obtain 113 g of the title compound as a as a pale brown colored solid with HPLC purity of 96.8%
References:
WO2021/152623,2021,A1 Location in patent:Paragraph 19; 20
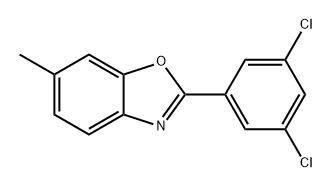
1414840-15-5
0 suppliers
inquiry

594839-88-0
153 suppliers
$59.00/5mg


