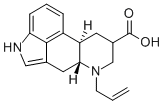
6-Allyl-8beta-carboxyergoline synthesis
- Product Name:6-Allyl-8beta-carboxyergoline
- CAS Number:81409-74-7
- Molecular formula:C18H20N2O2
- Molecular Weight:296.36
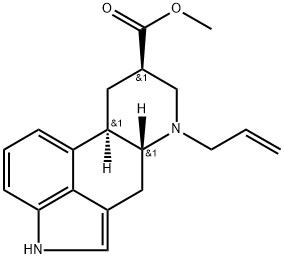
72821-79-5
39 suppliers
inquiry

81409-74-7
49 suppliers
inquiry
Yield: 85%
Reaction Conditions:
Stage #1:methyl (6aR,9R,10aR)-7-allyl-4,6,6a,7,8,9,10,10a-octahydroindolo[4,3-fg]quinoline-9-carboxylate with sodium hydroxide;water in methanol at 20 - 30; for 2 - 3 h;
Stage #2: with hydrogenchloride in methanol;water; pH=6 - 6.5 at 0 - 5; for 1 - 2 h;
Steps:
10
To a mixture of methyl ergoline-8μ-carboxylate hydrochloride (185 g) and triethylamine (183.3 g) in N,N-dimethylformide (555 mL) is added allyl bromide (146.3 g). The mixture is stirred at 20-30° C. for 4-5 h or until the reaction completion. The reaction mixture is then cooled to 0-5° C. and to it is added water (1.3 L) in portions. The resulting suspension is stirred at 0-5° C. for 1-3 h, then is filtered and washed with N,N-dimethylformide/water (1:2 v/v) and then water to give methyl 6-(2-propenyl)-ergoline-8β-carboxylate.The damp product is mixed with methanol (925 mL) and to it is added 50% sodium hydroxide solution (63 g). After stirring at 20-30° C. for 2-3 h, water (500 mL) is added and the pH of the mixture is adjusted to 6-6.5 by the slow addition of hydrochloric acid. The resulting suspension is stirred at 0-5° C. for 1-2 h, then filtered, washed with water and methanol. The isolated solid is dried to give 6-(2-propenyl)-ergoline-8β-carboxylic acid (152.3 g) in 85% yield.
References:
Apotex Pharmachem Inc. US2008/275240, 2008, A1 Location in patent:Page/Page column 11-12
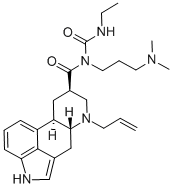
![(82)-N-[[[3-(Dimethylamino)propyl]amino]carbonyl]-N-ethyl-6-(2-propen-1-yl)-ergoline-8-carboxamide](/CAS/20211123/GIF/81409-91-8.gif)
81409-91-8
13 suppliers
inquiry

81409-74-7
49 suppliers
inquiry
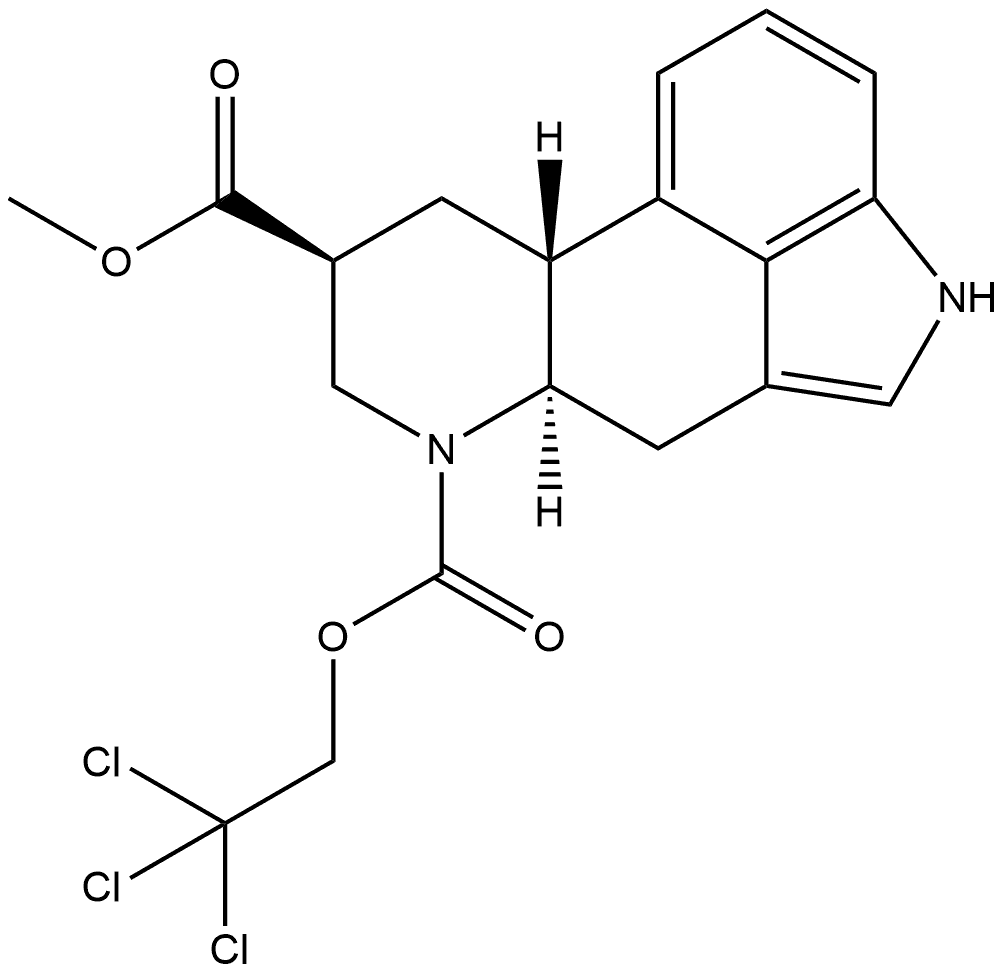
81409-90-7
321 suppliers
$35.00/1mg

81409-74-7
49 suppliers
inquiry
80993-64-2
2 suppliers
inquiry

81409-74-7
49 suppliers
inquiry
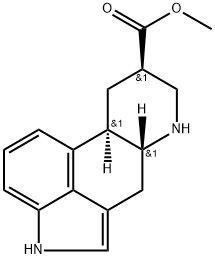
30341-92-5
17 suppliers
inquiry

81409-74-7
49 suppliers
inquiry