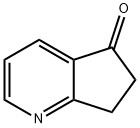
6,7-DIHYDRO-5H-1-PYRIDIN-5-ONE synthesis
- Product Name:6,7-DIHYDRO-5H-1-PYRIDIN-5-ONE
- CAS Number:28566-14-5
- Molecular formula:C8H7NO
- Molecular Weight:133.15
![Cyclopenta[b]pyridine](/CAS/GIF/533-37-9.gif)
533-37-9

28566-14-5
The general procedure for the synthesis of 6,7-dihydrocyclopenta[b]pyridin-5-one using 2,3-cyclopentenopyridine as starting material was as follows: 0.883 mg of Mn(OTf)2 (0.5 mol%), 60 mg of 2,3-cyclopentenopyridine, 0.35 g of 65% aqueous TBHP and 2.5 mL of water were added sequentially to 25 mL of a round bottomed flask. The reaction system was stirred in air at room temperature for 24 hours. Upon completion of the reaction, the reaction mixture was extracted with ethyl acetate (3 × 5 mL), the organic phases were combined and dried over anhydrous sodium sulfate. After filtration, the solvent was removed by distillation under reduced pressure. The crude product was purified by silica gel column chromatography using petroleum ether and ethyl acetate (5:1, v/v) as eluent to afford 60.5 mg of 6,7-dihydro-5H-cyclopentadieno[b]pyridin-5-one as a light yellow solid in 91% yield.
![Cyclopenta[b]pyridine](/CAS/GIF/533-37-9.gif)
533-37-9
342 suppliers
$6.00/5g

28566-14-5
154 suppliers
$31.00/100mg
Yield:28566-14-5 91%
Reaction Conditions:
with tert.-butylhydroperoxide;manganese(II) triflate in water at 20; for 24 h;
Steps:
1 Example 1 Synthesis of 6,7-dihydro-5H-cyclopenta [b] pyridin-5-one
A solution of 0.883 mg of Mn (OTf) 2 (0.5 mol%), 60 mg of 2,3-cyclopentenopyridine, 0.35 g of 65% aqueous TBHP and 2.5 ml of water was added successively to a 25 mL round bottom flask, in the air at room temperature for 24 hours, the reaction solution was extracted with 3 x 5 mL of ethyl acetate, the ethyl acetate layer was collected, dried over anhydrous sodium sulfate, filtered and the ethyl acetate was distilled off, using petroleum ether and ethyl acetate in a volume ratio of 5: 1 as eluent, the product of 6,7-dihydro-5H-cyclopenta [b] pyridin-5-one 60.5 mg was obtained as a pale yellow solid in a yield of 91% by silica gel column chromatography.
References:
Dalian Institute of Chemical Physics, Chinese Academy of Sciences;Gao, Shuang;Ren, Lanhui;Wang, Lianyue;Lv, Ying;Zhang, Yi CN105669548, 2016, A Location in patent:Paragraph 0027; 0028
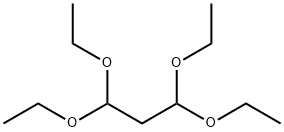
122-31-6
290 suppliers
$21.00/25mL
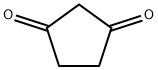
3859-41-4
306 suppliers
$6.00/1g

28566-14-5
154 suppliers
$31.00/100mg

624-67-9
62 suppliers
$120.00/500mg
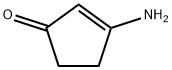
28566-12-3
27 suppliers
$45.00/10mg

28566-14-5
154 suppliers
$31.00/100mg