6,7-dihydro-5H-cyclopenta[b]pyridin-4-amine synthesis
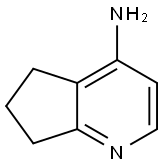
- Product Name:6,7-dihydro-5H-cyclopenta[b]pyridin-4-amine
- CAS Number:78183-15-0
- Molecular formula:C8H10N2
- Molecular Weight:134.18
![4-Nitro-6,7-dihydro-5H-cyclopenta[b]pyridine 1-oxide](/CAS/20150408/GIF/126259-73-2.gif)
126259-73-2
4 suppliers
inquiry
![6,7-dihydro-5H-cyclopenta[b]pyridin-4-amine](/CAS/GIF/78183-15-0.gif)
78183-15-0
26 suppliers
$94.00/50mg
Yield:78183-15-0 380 mg
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in methanol; under 1500.15 Torr; for 24 h;
Steps:
2.2-c Step 2-c: Preparation of 6,7-dihydro-5H-cvclopentarb1pyridin-4-amine:
To a stirred solution of 4-nitro-6,7-dihydro-5H-cyclopenta[b]pyridine-l -oxide (640 mg; 3.37 mmol) in MeOH (20 mL) was added 10% palladium on activated carbon (359 mg; 0.34 mmol). The reaction solution was stirred under hydrogen atmosphere (2 bars) for 24 h. Insolubles were removed by filtration and the cake was washed with MeOH. The combined filtrate was concentrated to dryness to afford 6,7- dihydro-5H-cyclopenta[b]pyridin-4-amine (380 mg) as a light brown solid. (0729) MS m/z (+ESI): 135.2 [M+H]+. (0730) 'H-NMR (400 MHz, DMSO-i δ ppm: 7.77 (d, J = 5.5 Hz, 1H), 6.27 (d, J= 5.5 Hz, 1H), 5.71 (s, 2H), 2.73 (t, J= 7.7 Hz, 2H), 2.64 (t, J = 7.4 Hz, 2H), 1.99 - 1.92 (m, 2H).
References:
WO2019/72978,2019,A1 Location in patent:Page/Page column 76
![Cyclopenta[b]pyridine](/CAS/GIF/533-37-9.gif)
533-37-9
330 suppliers
$6.00/5g
![6,7-dihydro-5H-cyclopenta[b]pyridin-4-amine](/CAS/GIF/78183-15-0.gif)
78183-15-0
26 suppliers
$94.00/50mg
![6,7-Dihydro-5H-cyclopenta[b]pyridine 1-oxide](/CAS/GIF/90685-58-8.gif)
90685-58-8
29 suppliers
$45.00/50mg
![6,7-dihydro-5H-cyclopenta[b]pyridin-4-amine](/CAS/GIF/78183-15-0.gif)
78183-15-0
26 suppliers
$94.00/50mg