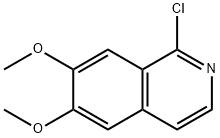
6,7-DiMethoxy-1-chloroisoquinoline synthesis
- Product Name:6,7-DiMethoxy-1-chloroisoquinoline
- CAS Number:21560-29-2
- Molecular formula:C11H10ClNO2
- Molecular Weight:223.66
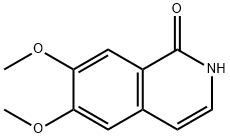
16101-63-6

21560-29-2
General procedure for the synthesis of 1-chloro-6,7-dimethoxyisoquinolin-1(2H)-one from 6,7-dimethoxyisoquinolin-1(2H)-one: a mixed solution of 6,7-dimethoxyisoquinolin-1(2H)-one (200 mg, 0.97 mmol) and phosphorus trichloride (0.268 mL, 2.92 mmol) in acetonitrile (5 mL) was placed in a microwave reactor at 110 °C for 5 min with stirring. Upon completion of the reaction, the reaction was quenched with saturated aqueous sodium bicarbonate solution and stirring was continued for 1 h at room temperature. The reaction mixture was filtered through a diatomaceous earth pad and washed with ethyl acetate. The filtrate was concentrated to dryness under reduced pressure. The crude product was purified by fast column chromatography with the eluent being a solvent mixture of heptane and ethyl acetate (the ratio was tapered from 1:0 to 0:1) to afford the target product, 1-chloro-6,7-dimethoxyisoquinoline, as a gel (99 mg, 45.4% yield). Mass spectrometry analysis showed m/z 224.0 [M + H]+ (ESI).

16101-63-6
28 suppliers
$45.00/50mg

21560-29-2
22 suppliers
$74.00/50mg
Yield:21560-29-2 92%
Reaction Conditions:
with trichlorophosphate at 100; for 0.5 h;Inert atmosphere;Microwave irradiation;
References:
Janssen-Müller, Daniel;Singha, Santanu;Lied, Fabian;Gottschalk, Karin;Glorius, Frank [Angewandte Chemie - International Edition,2017,vol. 56,# 22,p. 6276 - 6279][Angew. Chem.,2017,vol. 129,# 22,p. 6373 - 6376,4] Location in patent:supporting information
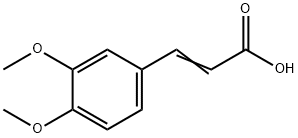
2316-26-9
401 suppliers
$10.00/5g

21560-29-2
22 suppliers
$74.00/50mg
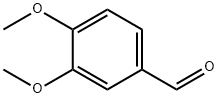
120-14-9
808 suppliers
$5.00/25g

21560-29-2
22 suppliers
$74.00/50mg

24186-50-3
0 suppliers
inquiry

21560-29-2
22 suppliers
$74.00/50mg

24186-43-4
0 suppliers
inquiry

21560-29-2
22 suppliers
$74.00/50mg