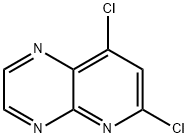
6,8-dichloropyrido[2,3-b]pyrazine synthesis
- Product Name:6,8-dichloropyrido[2,3-b]pyrazine
- CAS Number:1283075-60-4
- Molecular formula:C7H3Cl2N3
- Molecular Weight:200.02

131543-46-9
1 suppliers
inquiry
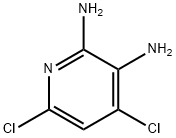
24484-99-9
41 suppliers
$45.00/2.5mg
![6,8-dichloropyrido[2,3-b]pyrazine](/CAS/20180629/GIF/1283075-60-4.gif)
1283075-60-4
19 suppliers
inquiry
Yield:-
Reaction Conditions:
in tetrahydrofuran;water at 20; for 72 h;
Steps:
1.1
A solution of of 4.81 g (27 mmol) 4,6-dichloro-pyridine-2,3-diamine (synthesis described in J. E. Schelling, C. A. Salemink, Rec. Trav. Chim. Pays-Bas 91 , 650 [1972]) in 50 ml THF was treated with 5.22 g (27 mmol) of a 30% solution of glyoxal in water and the mixture was stirred for 3 days at room temperature. The reaction mixture was evaporated and the residue was partitioned between dichloromethane and diluted sodium carbonate solution. The organic phase was dried over sodium sulfate and evaporated. The residue was chromatographed on a silica gel column with cyclohexane/ethyl acetate as eluent yielding 6,8-dichloro- pyrido[2,3-b]pyrazine as off-white fine needles; HPLC/MS (B): 1.93 min, [M+H] 200. 1H NMR (400 MHz, CDCI3) δ = 9.16 (d, J=1.7, 1 H), 9.05 (d, J=1.7, 1H), 7.89 (s, 1 H).
References:
MERCK PATENT GMBH;DORSCH, Dieter;JONCZYK, Alfred;HOELZEMANN, Guenter;AMENDT, Christiane;ZENKE, Frank WO2012/119690, 2012, A1 Location in patent:Page/Page column 54
5049-61-6
444 suppliers
$8.00/10g
1663-67-8
225 suppliers
$36.79/5g
![6,8-dichloropyrido[2,3-b]pyrazine](/CAS/20180629/GIF/1283075-60-4.gif)
1283075-60-4
19 suppliers
inquiry