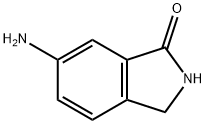
6-AMino-2,3-dihydroisoindol-1-one synthesis
- Product Name:6-AMino-2,3-dihydroisoindol-1-one
- CAS Number:675109-45-2
- Molecular formula:C8H8N2O
- Molecular Weight:148.16
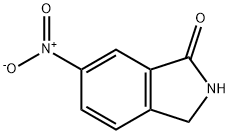
110568-64-4

675109-45-2
General procedure for the synthesis of 6-amino-isoindolin-1-one from 6-nitro-isoindolin-1-one: A suspension of intermediate 6B (1.6 g, 8.98 mmol) and Pd/C (0.18 g) in methanol (100 mL) was stirred for 4 hr under hydrogen (1 atm) atmosphere. After completion of the reaction, the reaction mixture was filtered and the filter cake was washed with methanol. After combining the filtrates, the solvent was removed by concentration under reduced pressure. The crude product was ground with methanol (10 mL) and subsequently dried under reduced pressure to afford intermediate 6 (800 mg, 5.40 mmol, 60.1% yield) as a beige solid. The structure of the product was confirmed by 1H NMR (400 MHz, DMSO-d6): δ 4.15 (s, 2H), 5.26 (s, 2H), 6.77 (dd, J = 8.25, 2.20 Hz, 1H), 6.80 (s, 1H), 7.16 (d, J = 8.79 Hz, 1H), 8.29 (s, 1H). The mass spectrum (ESI) showed m/z 149.2 ([M + H]+).

110568-64-4
128 suppliers
$24.00/100mg

675109-45-2
110 suppliers
$165.00/100mg
Yield:675109-45-2 60.1%
Reaction Conditions:
with hydrogen;palladium on activated charcoal in methanol under 760.051 Torr; for 4 h;
Steps:
6
Intermediate 6[00204] A suspension of Intermediate 6B (1.6 g, 8.98 mmol) and Pd/C (0.18 g) in MeOH (100 mL) was stirred under H2 (1 atm) for 4 h. The reaction mixture was filtered and the filter cake was washed with MeOH. The combined filtrates were
References:
BRISTOL-MYERS SQUIBB COMPANY WO2008/79759, 2008, A1 Location in patent:Page/Page column 91-92
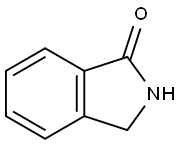
480-91-1
185 suppliers
$21.00/500 mg

675109-45-2
110 suppliers
$165.00/100mg
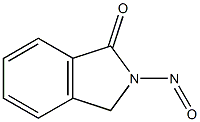
5415-18-9
1 suppliers
inquiry

675109-45-2
110 suppliers
$165.00/100mg
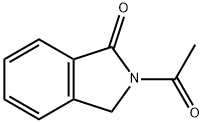
3006-64-2
1 suppliers
inquiry

675109-45-2
110 suppliers
$165.00/100mg