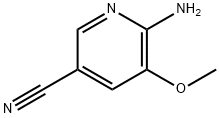
6-Amino-5-methoxy-nicotinonitrile synthesis
- Product Name:6-Amino-5-methoxy-nicotinonitrile
- CAS Number:1256821-98-3
- Molecular formula:C7H7N3O
- Molecular Weight:149.15
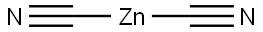
557-21-1
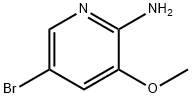
42409-58-5

1256821-98-3
Under nitrogen protection, 5-bromo-3-methoxypyridin-2-amine (3.00 g, 14.8 mmol) was dissolved in N,N-dimethylformamide (55 mL), and zinc(II) cyanide (2.78 g, 23.6 mmol) and tetrakis(triphenylphosphine)palladium(0) (2.06 g, 1.77 mmol) were added sequentially. The reaction mixture was stirred at 100 °C for 4 hours. After completion of the reaction, the reaction mixture was diluted with ethyl acetate and washed sequentially with saturated ammonium hydroxide solution and brine. The organic and aqueous phases were separated, and the organic phase was dried with anhydrous sodium sulfate and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (eluent ratio: ethyl acetate/cyclohexane=1:5) to afford 6-amino-5-methoxypyridine-3-carbonitrile (1.2 g, 54% yield). The product characterization data were as follows: 1H NMR (400 MHz, CDCl3) δ 7.99 (s, 1H), 6.99 (s, 1H), 3.88 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 152.83, 144.58, 141.27, 118.32, 115.52, 97.66, 55.65; LC-MS (Method B): retention time 0.69 min, m/z 150 ([M+H]+).
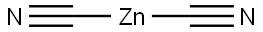
557-21-1
106 suppliers
$12.00/5g

42409-58-5
109 suppliers
$8.00/100mg

1256821-98-3
18 suppliers
inquiry
Yield:1256821-98-3 54%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0) in N,N-dimethyl-formamide at 100; for 4 h;Inert atmosphere;
Steps:
I Example I: Preparation of 6-amino-5-methoxy-pyridine-3-carbonitrile
To a solution of 5-bromo-3-methoxy-pyridin-2-amine (3.00 g, 14.8 mmol) in N,N- dimethylformamide (55 mL) under a nitrogen atmosphere was added zinc (II) cyanide (2.78 g, 23.6 mmol) and tetrakis(triphenylphosphine)palladium(0) (2.06 g, 1.77 mmol). The reaction mixture was stirred at 100°C for 4 h. The reaction mixture was diluted with ethyl acetate and washed successively with a saturated solution of ammonium hydroxide and brine. The phases were separated. The organic phases was dried over sodium sulfate and concentrated. The residue was purified by column chromatography on silica gel (eluent: ethyl acetate / cyclohexane 1 :5) to give 6-amino-5-methoxy-pyridine-3-carbonitrile (1.2 g, 54% yield).1H NMR (400 MHz, CDC13): 7.99 (s, 1H), 6.99 (s, 1H), 3.88 (s, 3H) ppm. 13C NMR (100 MHz, CDC13): 152.83, 144.58, 141.27, 118.32, 115.52, 97.66, 55.65. LC-MS (Method B): RT 0.69, 150 (M+H+)
References:
WO2013/37755,2013,A1 Location in patent:Page/Page column 24