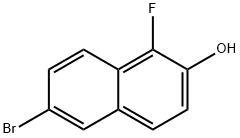
6-bromo-1-fluoronaphthalen-2-ol synthesis
- Product Name:6-bromo-1-fluoronaphthalen-2-ol
- CAS Number:442150-49-4
- Molecular formula:C10H6BrFO
- Molecular Weight:241.06
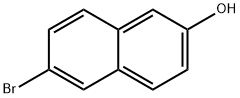
15231-91-1

442150-49-4
Under argon protection, 4.46 g (0.02 mol) of 6-bromo-2-naphthol with 250 mL of acetonitrile was added to a 250 mL three-necked flask and stirred until completely dissolved. Subsequently, 8.50 g (0.024 mol) of Selectfluor was added to the solution. the reaction mixture was heated to 60 °C with continuous stirring for 12 h. The reaction was completed by pouring the reaction mixture into the flask. Upon completion of the reaction, the mixture was poured into 100 mL of water and extracted by adding 150 mL of ethyl acetate. After stirring, the mixture was left to stratify and the organic phase was separated. The organic phase was washed three times with water, dried with anhydrous sodium sulfate, filtered and concentrated. The crude product was purified by silica gel column chromatography, and the eluent was a mixed system of petroleum ether/ethyl acetate. The target fraction was collected and the solvent was removed by rotary evaporation to give a yellow solid. After standing at room temperature, a large amount of yellow solid was precipitated, filtered and dried to give 2.64 g of 6-bromo-1-fluoro-2-naphthol in 54.6% yield.

15231-91-1
405 suppliers
$7.00/5g

442150-49-4
31 suppliers
$215.00/100mg
Yield:442150-49-4 88%
Reaction Conditions:
Stage #1: 6-bromo-naphthalen-2-olwith Selectfluor in acetonitrile at 20; for 2 h;
Stage #2: with sodium dithionite in water;isopropyl alcohol;toluene at 20; for 24 h;Inert atmosphere;
Steps:
General procedure A
General procedure: To a solution of substituted naphthalen-2-ol (10 mmol) in MeCN (100 mL) was added the 1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) (20 mmol) in roomtemperature. The resulting mixture was stirred for 2 h and then added the water, the organic layer wasseparated and the aqueous layer was extracted with ethyl acetate, and the combined organic layer was driedover anhydrous MgSO4 and concentrated under reduced pressure. The crude product was directly used inthe next step without purification.To a round-bottom flask was added the Na2S2O4 (25 mmol) in iPrOH (50 mL) and H2O (50 mL) atroom temperature under argon. Then the above obtained crude product in toluene (100 mL) at roomtemperature under argon was added to the round-bottom flask dropwise. The resulting mixture was stirredfor 24 h under argon and then added the water, the organic layer was separated and the aqueous layer wasS3extracted with ethyl acetate, and the combined organic layer was dried over anhydrous MgSO4 andconcentrated under reduced pressure. The residue was then chromatographed on silica gel to afford thesubstituted 1-fluoronaphthalen-2-ol as a solid.
References:
Zhang, Naichen;Ye, Yuanzhi;Bai, Lu;Liu, Jingjing;Wang, Han;Luan, Xinjun [Chinese Chemical Letters,2022,vol. 33,# 5,p. 2411 - 2414] Location in patent:supporting information
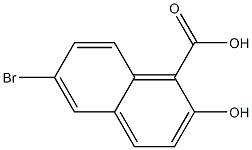
65726-23-0
3 suppliers
inquiry

442150-49-4
31 suppliers
$215.00/100mg