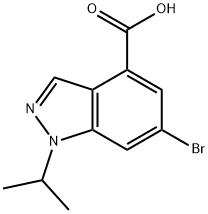
6-BROMO-1-ISOPROPYL-1H-INDAZOLE-4-CARBOXYLIC ACID synthesis
- Product Name:6-BROMO-1-ISOPROPYL-1H-INDAZOLE-4-CARBOXYLIC ACID
- CAS Number:1346702-54-2
- Molecular formula:C11H11BrN2O2
- Molecular Weight:283.12
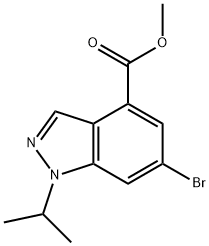
1346702-52-0

1346702-54-2
b) Methyl 6-bromo-1-isopropyl-1H-indazole-4-carboxylate (0.58 g, 1.952 mmol) was dissolved in a solvent mixture of methanol (12 mL) and tetrahydrofuran (3 mL). A 3N NaOH solution (3.25 mL, 9.76 mmol) was added slowly via syringe and the reaction mixture was stirred at room temperature for 3 hours. Upon completion of the reaction, the volatile solvent was removed under vacuum and the residue was diluted with water and the pH was adjusted to 4-5 by slow dropwise addition of 1 N HCl. The aqueous phase was extracted with a 20% THF/EtOAc solvent mixture (3 times). The organic phases were combined, washed with saturated brine, dried over anhydrous MgSO4, filtered and concentrated to afford 6-bromo-1-isopropyl-1H-indazole-4-carboxylic acid (0.52 g, 94% yield). The product was characterized by 1H NMR (400 MHz, DMSO-d6) and LC-MS (ES): 1H NMR δ 13.54 (s, 1H), 8.36-8.43 (m, 2H), 7.82 (d, J = 1.52 Hz, 1H), 5.11 (quin, J = 6.57 Hz, 1H), 1.48 (s, 3H), 1.47 ( s, 3H); LC-MS (ES) [M + H]+ 283.4/285.2.

1346702-52-0
38 suppliers
$45.00/10mg

1346702-54-2
37 suppliers
$80.00/100mg
Yield:1346702-54-2 94%
Reaction Conditions:
Stage #1: methyl 6-bromo-1-(1-methylethyl)-1H-indazole-4-carboxylatewith water;sodium hydroxide in tetrahydrofuran;methanol at 20; for 3 h;
Stage #2: with hydrogenchloride in water; pH=4 - 5;
Steps:
3.b
b) 6-bromo-l-(l-methylethyl)-l//-indazole-4-carboxylic acidTo a solution of 6-bromo-l-(l-methylethyl)-lH-indazole-4-carboxylate (0.58 g, 1.952 mmol in methanol (12 mL) and tetrahydrofuran (3 mL) was added 3 N NaOH (3.25 mL, 9.76 mmol) via syringe and stirred for 3 h at RT. The volatiles were removed in vacuo then diluted with water and slowly acidifed with 1 N HC1 to pH 4-5. The contents were extracted with 20% THF/EtOAc (3x). The combined organics were washed with brine, dried over MgS04, filtered, and concentrated to give 6-bromo-l-(l-methylethyl)-lH-indazole-4-carboxylic acid (0.52 g, 94 % yield). H NMR (400 MHz, DMSO-i/6) d ppm 13.54 (s, 1 H) 8.36 - 8.43 (m, 2 H) 7.82 (d, J=1.52 Hz, 1 H) 5.11 (quin, J=6.57 Hz, 1 H) 1.48 (s, 3 H) 1.47 (s, 3 H); LC-MS(ES) [M+H]+ 283.4/285.2
References:
WO2011/140325,2011,A1 Location in patent:Page/Page column 41

885518-49-0
139 suppliers
$6.00/250mg

1346702-54-2
37 suppliers
$80.00/100mg
107650-20-4
158 suppliers
$9.00/250mg

1346702-54-2
37 suppliers
$80.00/100mg
220514-28-3
149 suppliers
$10.00/1g

1346702-54-2
37 suppliers
$80.00/100mg
1975-50-4
467 suppliers
$18.00/25g

1346702-54-2
37 suppliers
$80.00/100mg