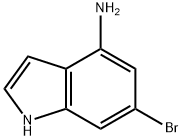
6-Bromo-1H-indol-4-amine synthesis
- Product Name:6-Bromo-1H-indol-4-amine
- CAS Number:350800-81-6
- Molecular formula:C8H7BrN2
- Molecular Weight:211.06
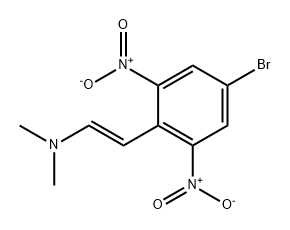
350800-78-1

350800-81-6
To 6-bromo-1H-indol-4-amine (3 g, 9.49 mmol) was added HCl solution (61.20 g, 167.86 mmol) of TiCl3 (7.32 g, 47.45 mmol). The reaction mixture was stirred at 25 °C for 16 hours. After completion of the reaction, the mixture was slowly poured into 2N aqueous NaOH solution (150 mL) and extracted with EtOAc (150 mL). The organic phase was dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (eluent: petroleum ether to petroleum ether/ethyl acetate = 5/1) to afford 6-bromo-1H-indol-4-amine (2 g, 36% yield) as a gray solid.1H NMR (CDCl3, 400 MHz) δ 8.10 (br s, 1H), 7.09-7.08 (m, 1H), 7.02 (d, J = 1.4Hz , 1H), 6.54 (d, J = 1.4Hz, 1H), 6.44-6.43 (m, 1H), 3.98 (br s, 2H).

350800-78-1
0 suppliers
inquiry

350800-81-6
82 suppliers
$45.00/10mg
Yield:350800-81-6 36%
Reaction Conditions:
with hydrogenchloride;titanium(III) chloride in water at 25; for 16 h;
Steps:
17.2 Step 2: 6-bromo-lH-indol-4-amine
The title compound from step 1 (3 g, 9.49 mmol) was treated with T1CI3 (7.32 g, 47.45 mmol) in a HCl solution (61.20 g, 167.86 mmol). The reaction mixture was stirred at 25 °C for 16 hr. It was then poured over 2 N aqueous NaOH (150 mL) and extracted with EtOAc (150 mL). The organic phase was dried, filtered and concentrated under reduced pressure. The residue was purified by column chromatography (PE to PE/EA=5/1) to give the title compound (2 g, 36%) as a gray solid. H NMR (CDC13, 400 MHz) δ 8.10 (br s, 1 H ), 7.09-7.08 (m, 1 H), 7.02 (d, J = 1.4 Hz, 1 H), 6.54 (d, J = 1.4 Hz, 1 H), 6.44-6.43 (m, 1 H), 3.98 (br s, 2H).
References:
WO2016/168682,2016,A2 Location in patent:Paragraph 0256
95192-64-6
106 suppliers
$19.00/250mg

350800-81-6
82 suppliers
$45.00/10mg
16533-71-4
221 suppliers
$9.00/10g

350800-81-6
82 suppliers
$45.00/10mg
885519-01-7
68 suppliers
$45.00/10mg

350800-81-6
82 suppliers
$45.00/10mg