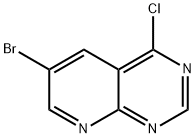
Pyrido[2,3-d]pyrimidine, 6-bromo-4-chloro- synthesis
- Product Name:Pyrido[2,3-d]pyrimidine, 6-bromo-4-chloro-
- CAS Number:1215787-31-7
- Molecular formula:C7H3BrClN3
- Molecular Weight:244.48
![6-BROMOPYRIDO[2,3-D]PYRIMIDIN-4(1H)-ONE](/CAS/GIF/155690-79-2.gif)
155690-79-2
![Pyrido[2,3-d]pyrimidine, 6-bromo-4-chloro-](/CAS/GIF/1215787-31-7.gif)
1215787-31-7
General procedure for the synthesis of 6-bromo-4-chloropyrido[2,3-d]pyrimidin-4(1H)-one from 6-bromopyrido[2,3-d]pyrimidin-4(1H)-one: 6-bromopyrido[2,3-d]pyrimidin-4(1H)-one (3.0 g) was mixed with phosphorus trichloride (POCl3, 15 mL) and heated to reflux for 3 hours at 130 °C under nitrogen atmosphere. Upon completion of the reaction, the dark homogeneous solution was concentrated under reduced pressure and diluted with ethyl acetate (EtOAc, 150 mL). The resulting brown non-homogeneous slurry was slowly poured into an ice-water mixture, neutralized to pH neutrality by the addition of sodium bicarbonate (NaHCO3), and the mixture was allowed to gradually warm to room temperature. Subsequently, the organic layer was separated by further diluting the mixture with ethyl acetate (75 mL). The organic layer was washed sequentially with saturated aqueous sodium chloride (NaCl), dried over anhydrous magnesium sulfate (MgSO4) and filtered through a diatomaceous earth pad and silica gel column. The filtrate was concentrated to give a light yellow crude product, which was recrystallized by stirring in a 50% ethyl acetate/hexane solvent mixture. After filtration, 6-bromo-4-chloropyrido[2,3-d]pyrimidine was obtained as a light yellow crystalline solid (1.8 g, purity: 95%).
![6-BROMOPYRIDO[2,3-D]PYRIMIDIN-4(1H)-ONE](/CAS/GIF/155690-79-2.gif)
155690-79-2
64 suppliers
$45.00/10mg
![Pyrido[2,3-d]pyrimidine, 6-bromo-4-chloro-](/CAS/GIF/1215787-31-7.gif)
1215787-31-7
65 suppliers
$65.00/100mg
Yield:1215787-31-7 80.9%
Reaction Conditions:
with triethylamine;trichlorophosphate in toluene at 0 - 110; for 3 h;
Steps:
2.1.5. Synthesis 6-bromo-4-chloropyrido[2,3-d]pyrimidine(7)
The compound 6 (20 g, one eq) and toluene (100 mL) were added to a 250 mL flask with stirring, followed by phosphorus oxychloride (22.75 mL, two eq) and triethylamine (37.33 mL, 2.Two eq) were slowly added at 0 °C. The reaction mixture was stirred for 3 h at 110 °C. The reaction solution was poured into ice water to separate out solids, filtered, the filter cake was washed with ethyl acetate (50 mL 3), the filtrate was extracted with ethyl acetate (100 mL 3), the combined organic layer was dried over anhydrous Na2SO4, concentrated to remove the organic phase, and the residue was dried to give the compound 7 (17.50 g, yield 80.9%).
References:
Sun, Hong;Wang, Zhongyuan;Deng, Liyuan;Hu, Weiyin;Liao, Tianhui;Zhao, Xin;Zhou, Zhixu;Chai, Huifang;Zhao, Chunshen [Molecular Crystals and Liquid Crystals,2022,vol. 742,# 1,p. 10 - 24]
![6-BROMOPYRIDO[2,3-D]PYRIMIDIN-4(1H)-ONE](/CAS/GIF/155690-79-2.gif)
155690-79-2
64 suppliers
$45.00/10mg
![Pyrido[2,3-d]pyrimidine, 6-bromo-4-chloro-](/CAS/GIF/1215787-31-7.gif)
1215787-31-7
65 suppliers
$65.00/100mg
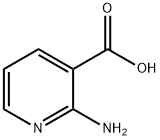
5345-47-1
393 suppliers
$9.00/1g
![Pyrido[2,3-d]pyrimidine, 6-bromo-4-chloro-](/CAS/GIF/1215787-31-7.gif)
1215787-31-7
65 suppliers
$65.00/100mg
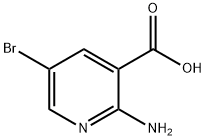
52833-94-0
217 suppliers
$13.00/1g
![Pyrido[2,3-d]pyrimidine, 6-bromo-4-chloro-](/CAS/GIF/1215787-31-7.gif)
1215787-31-7
65 suppliers
$65.00/100mg
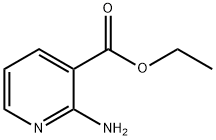
13362-26-0
212 suppliers
$7.00/1g
![Pyrido[2,3-d]pyrimidine, 6-bromo-4-chloro-](/CAS/GIF/1215787-31-7.gif)
1215787-31-7
65 suppliers
$65.00/100mg