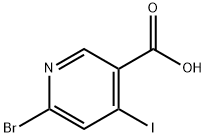
6-broMo-5-iodonicotinic acid synthesis
- Product Name:6-broMo-5-iodonicotinic acid
- CAS Number:1200130-82-0
- Molecular formula:C6H3BrINO2
- Molecular Weight:327.9
6311-35-9
369 suppliers
$15.00/1g

1200130-82-0
18 suppliers
inquiry
Yield:1200130-82-0 70%
Reaction Conditions:
Stage #1: 6-bromopyridine-3-carboxylic acidwith 2,2,6,6-tetramethyl-piperidine;n-butyllithium in tetrahydrofuran;hexane at -20; for 2 h;Inert atmosphere;
Stage #2: with iodine in tetrahydrofuran at -50 - 20;Inert atmosphere;
Steps:
1.1 Step 1 : 6-bromo-4-iodonicotinic acid
A dry three neck-round bottom flask (10 L) contained TMP (2,2,6,6-tertramethylpiperidine, fresh distilled, 629 g, 4.46 mol) and dry THF (3 L) under N2 was cooled to below -55°C. n-BuLi in hexane solution (2.5 M, 1783 mL, 4.46 mol) was added dropwise to the cooled solution over 2 hrs. The suspension was stirred for 30 mins at below -40°C. 6-bromonicotinic acid (white powder, dried under vacuum before use) (300 g, 1.485 mol) was added quickly in four portions and the suspension was stirred at -20°C for 2 hrs, over which time the reaction turned from orange to brown. The reaction mixture was cooled to below -50 °C and quickly transferred into a pre-cooled solution of I2 (1130.8 g, 4.455 mol) in dry THF (3 L) under N2. The resulting mixture was allowed to warm to room temperature with stirring overnight. The reaction solvent was removed under vacuum and the residue was suspended in water (3000 mL), then washed with DCM (9 L x 2). The aqueous layer was acidified by addition of concentrated HC1 to pH = 2 and the precipitate was collected by filtration, washed with water (600 mL) and petroleum ether (300 mL), then dried under vacuum at 60 °C to afford the desired product as a tan solid (340 g, 70%).
References:
WO2020/23551,2020,A1 Location in patent:Paragraph 0274