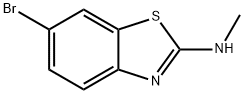
6-broMo-N-Methyl-1,3-benzothiazol-2-aMine synthesis
- Product Name:6-broMo-N-Methyl-1,3-benzothiazol-2-aMine
- CAS Number:75104-92-6
- Molecular formula:C8H7BrN2S
- Molecular Weight:243.12
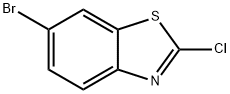
80945-86-4

74-89-5

75104-92-6
In a sealed reaction tube, 6-bromo-2-chlorobenzo[d]thiazole (701) (0.5 g, 2.0 mmol) was dissolved in methylamine solution (20 mL), and the resulting mixture was stirred and reacted at 60 °C overnight. Upon completion of the reaction, the mixture was cooled to room temperature, concentrated under reduced pressure to remove the solvent and quenched by the addition of water (20 mL). The precipitated solid was collected by filtration to afford the target product 6-bromo-N-methylbenzo[d]thiazol-2-amine (702) (0.43 g, 87.9% yield). The product was analyzed by ESI-MS and showed (M + H)+ m/z: 242.8.

80945-86-4
201 suppliers
$8.00/1g

74-89-5
0 suppliers
$13.44/25ML

75104-92-6
34 suppliers
$18.00/250mg
Yield:75104-92-6 87.9%
Reaction Conditions:
at 60;Sealed tube;
Steps:
5
[00394]6-Bromo-2-chlorobenzo[d]thiazole (701) (0.5 g, 2.0 mmol) was dissolved in methanamine solution (20 ml) in a sealed tube, and the resulting mixture was stirred at 60° C. overnight. The mixture was allowed to cool to RT, concentrated in vacuo and water (20 mL) was added. The solid was collected by filtration to afford the desired product, 6-bromo-N-methylbenzo[d]thiazol-2-amine (702) (0.43 g, 87.9% yield). ESI-MS (M+H)+m/z: 242.8.
References:
US2015/225407,2015,A1 Location in patent:Paragraph 0416
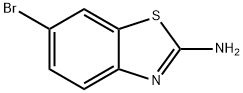
15864-32-1
237 suppliers
$6.00/1g

74-89-5
0 suppliers
$13.44/25ML

75104-92-6
34 suppliers
$18.00/250mg
61449-55-6
22 suppliers
inquiry

75104-92-6
34 suppliers
$18.00/250mg
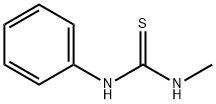
2724-69-8
81 suppliers
$22.00/1g

75104-92-6
34 suppliers
$18.00/250mg

106-40-1
484 suppliers
$12.00/5g

75104-92-6
34 suppliers
$18.00/250mg