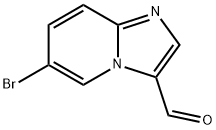
6-BROMOIMIDAZO[1,2-A]PYRIDINE-3-CARBALDEHYDE synthesis
- Product Name:6-BROMOIMIDAZO[1,2-A]PYRIDINE-3-CARBALDEHYDE
- CAS Number:30384-96-4
- Molecular formula:C8H5BrN2O
- Molecular Weight:225.04
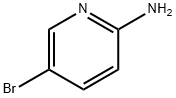
1072-97-5
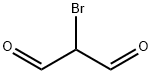
2065-75-0
![6-BROMOIMIDAZO[1,2-A]PYRIDINE-3-CARBALDEHYDE](/CAS/GIF/30384-96-4.gif)
30384-96-4
Step 1: 2-Bromomalonaldehyde (5230 mg, 34.68 mmol) was added to an acetonitrile solution of 2-amino-5-bromopyridine (5000 mg, 28.90 mmol). The reaction mixture was heated to reflux for 2 hours. After completion of the reaction, the reaction was quenched with saturated sodium bicarbonate solution and extracted with ethyl acetate (EtOAc). The organic layer was washed with brine and dried over anhydrous sodium sulfate. The organic layer was concentrated under reduced pressure, and the resulting crude product was purified by silica gel column chromatography using gradient elution with ethyl acetate-petroleum ether to afford 6-bromoimidazo[1,2-a]pyridine-3-carbaldehyde. Yield: 53%; 1H NMR (DMSO-d6, 300 MHz): δ 9.94 (s, 1H), 9.50 (s, 1H), 8.54 (s, 1H), 7.86-7.85 (m, 2H); MS (m/z): 226 (M + 1)+.

1072-97-5
702 suppliers
$9.00/1g

2065-75-0
338 suppliers
$6.00/5g
![6-BROMOIMIDAZO[1,2-A]PYRIDINE-3-CARBALDEHYDE](/CAS/GIF/30384-96-4.gif)
30384-96-4
99 suppliers
$13.00/100mg
Yield: 80%
Reaction Conditions:
in ethanol;water at 110; for 0.166667 h;Inert atmosphere;Microwave irradiation;Green chemistry;Solvent;Temperature;Time;
Steps:
General procedure for the synthesis of imidazo[1,2-a]pyridine-3-carbaldehyde (3)
General procedure: 6-bromo-2-aminopyridine (0.208 g, 1.2 mmol, 1 eq.) and 2-bromomalonaldehyde (0.272 g, 1.8 mmol, 1.5 eq.) were dissolved in the mixture of ethanol and water (v/v 1:1, total 2 ml) and placed in the pressure vial, equipped with a magnetic bar. The mixture was stirred for 1 min and purge with argon via syringe. Then microwave (MW) irradiation (with initial 150 W power) was applied. Depending on the substituents present in 2-aminopyridine 1, the following conditions were applied: a) for 2-aminopyridines substituted with halogen, or nitro or cyano groups: 10 min, 110 °C; or b) for 2-aminopyridines substituted with methyl or phenyl groups: 20 min, 100 °C. Afterwards, the reaction mixture was evaporated. Next the reaction mixture was neutralized with TEA, diluted with DCM (10 mL), and adsorbed on silica gel (~3 g).* The solvent was evaporated, and the residue was subjected to column chromatography using gradient DCM/acetone (100:0 => 75:25) as eluent to give product as yellow powder.
References:
Kusy, Damian;Maniukiewicz, Waldemar;Błażewska, Katarzyna M. [Tetrahedron Letters,2019,vol. 60,# 45,art. no. 151244] Location in patent:supporting information
107-20-0
0 suppliers
$18.10/5ml
138888-98-9
17 suppliers
inquiry
![6-BROMOIMIDAZO[1,2-A]PYRIDINE-3-CARBALDEHYDE](/CAS/GIF/30384-96-4.gif)
30384-96-4
99 suppliers
$13.00/100mg