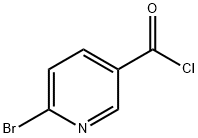
6-Bromonicotinoyl chloride synthesis
- Product Name:6-Bromonicotinoyl chloride
- CAS Number:740841-42-3
- Molecular formula:C6H3BrClNO
- Molecular Weight:220.45
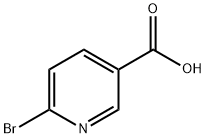
6311-35-9
374 suppliers
$15.00/1g

740841-42-3
10 suppliers
$279.23/1gm:
Yield:-
Reaction Conditions:
with thionyl chloride for 3 h;Reflux;
Steps:
4.A Step A: (6-bromopyridin-3 -yl (phenyl methanone (4-b)
6-bromopyridine-3-carboxylic acid (50g, 0.25mol) in thionyl chloride (4a, 130ml, 1.76mol) was heated at reflux for 3 hours. The mixture was then concentrated under vacuum and the residue was co-evaporated with benzene to remove the thionyl chloride. The resulting acid chloride was dissolved in benzene (120ml, 1.33mol) and treated with A1C13 (82.5g, 0.6mol) portion wise with stirring at 5 °C. After heating at reflux for 6 hours, the reaction mixture was cooled to room temperature and poured into 20% HC1 (500ml), stirred for lh, and layers were separated. The aqueous layer was further extracted with ethyl acetate (3x100 mL). The combined organic layer was washed with brine, 50%> KOH, and water (100 mL each), dried (Na2SC"4), filtered and concentrated under reduced pressure to afford the product, 4-b, (50g, 77%)
References:
WO2013/40790,2013,A1 Location in patent:Page/Page column 45