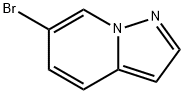
6-BroMopyrazolo[1,5-a]pyridine synthesis
- Product Name:6-BroMopyrazolo[1,5-a]pyridine
- CAS Number:1264193-11-4
- Molecular formula:C7H5BrN2
- Molecular Weight:197.03
![Ethyl 6-broMopyrazolo[1,5-a]pyridine-3-carboxylate](/CAS/20150408/GIF/55899-30-4.gif)
55899-30-4
![6-BroMopyrazolo[1,5-a]pyridine](/CAS/GIF/1264193-11-4.gif)
1264193-11-4
The general procedure for the synthesis of 6-bromopyrazolo[1,5-a]pyridine-3-carboxylic acid from ethyl 6-bromopyrazolo[1,5-a]pyridine is as follows: all reactions were carried out under decarboxylation/Vilsmeier conditions or hydrolysis/reduction/re-oxidation unless otherwise indicated. 1. Decarboxylation: Ethyl 6-bromopyrazolo[1,5-a]pyridine-3-carboxylate (1 equiv.) was dissolved in 40% aqueous H2SO4 (3 mL) and refluxed for 18 hours. Upon completion of the reaction, the solution was cooled in an ice bath, neutralized to pH 7 with 6 M NaOH, and then extracted twice with CH2Cl2. The organic phases were combined, dried with anhydrous Na2SO4 and concentrated under reduced pressure to give the decarboxylated product. 2. Vilsmeier reaction: Under the protection of nitrogen, the above decarboxylation product was dissolved in anhydrous DMF (2 mL), cooled to 0 °C, and POCl3 (3 equiv.) was added slowly. The reaction mixture was gradually warmed to room temperature and stirred for 2 hours. The reaction solution was poured into ice water, the pH was adjusted to 10 with 1 M NaOH and stirred for 1 h. The reaction solution was extracted twice with CH2Cl2. The organic phases were combined, washed twice with water, dried over anhydrous Na2SO4 and concentrated under reduced pressure to give the aldehyde product. Alternative route: 1. Hydrolysis of the ester: Ethyl 6-bromopyrazolo[1,5-a]pyridine-3-carboxylate (1 eq.) was dissolved in a mixture of 1 M NaOH (3 eq.) and EtOH (5 mL) and refluxed for 6 hours. EtOH was removed under reduced pressure and the residual aqueous phase was acidified to pH 1 with 1 M HCl, the precipitated carboxylic acid was collected by filtration, washed with water and dried. 2. Reduction of carboxylic acid: The above carboxylic acid (1 eq.) was suspended in anhydrous THF (10 mL) under nitrogen protection, CDI (1.5 eq.) was added, and stirred for 18 hours. The resulting solution was added dropwise to a solution of NaBH4 (5 eq.) in water (10 mL) and stirred for 30 min. The reaction solution was quenched with 1 M HCl and stirring was continued for 30 min. The solution was neutralized with saturated aqueous NaHCO3 and extracted twice with CH2Cl2. The organic phases were combined, dried over anhydrous Na2SO4, concentrated under reduced pressure, and purified by column chromatography (hexane:EtOAc gradient elution) to give the alcohol product. 3. Reoxidation of alcohols: A suspension of pyrazolo[1,5-a]pyridine-3-methanol (1 eq.) with MnO2 (10 eq.) in CH2Cl2 (2 mL) was stirred at room temperature for 4 days. The reaction mixture was filtered through diatomaceous earth, washed with CH2Cl2 and the filtrate was concentrated under reduced pressure to give the aldehyde product.
![Ethyl 6-broMopyrazolo[1,5-a]pyridine-3-carboxylate](/CAS/20150408/GIF/55899-30-4.gif)
55899-30-4
49 suppliers
$157.00/1 / G
![6-BroMopyrazolo[1,5-a]pyridine](/CAS/GIF/1264193-11-4.gif)
1264193-11-4
121 suppliers
$89.00/250mg
Yield:1264193-11-4 50%
Reaction Conditions:
Stage #1:ethyl 6-bromopyrazolo[1,5-a]pyridine-3-carboxylate with sulfuric acid;water for 18 h;Reflux;
Stage #2: with sodium hydroxide in water; pH=7
Steps:
4.1.2. Synthesis of pyrazolo[1,5-a]pyridine-3-carbaldehydes 4
General procedure: These were made by decarboxylation/Vilsmeier or hydrolysis/reduction/reoxidation as detailed below, unless otherwise stated.Decarboxylation was carried out by refluxing a solution of the ester (1 equiv) in 40% aqueous H2SO4 (3 mL) for 18 h. The solution was then cooled in ice and neutralised to pH 7 with 6 M NaOH, then extracted twice with CH2Cl2. The combined extracts were dried (Na2SO4) and the solvent removed in vacuo to leave the decarboxylated material. The pyrazolo[1,5-a]pyridine was then reacted under Vilsmeier conditions in dry DMF (2 mL) with POCl3 (3 equiv) at 0 °C under an atmosphere of N2. The reaction mixture was then warmed to room temperature and stirred for 2 h. The solution was poured onto ice, basified to pH 10 with 1 M NaOH, stirred for 1 h then extracted twice with CH2Cl2. The combined extracts were washed twice with water, dried (Na2SO4) and the solvent removed in vacuo to leave the aldehyde.Alternatively, the ester was hydrolysed by refluxing a solution of the ester (1 equiv) in 1 M NaOH (3 equiv) and EtOH (5 mL) for 6 h. The EtOH was removed in vacuo, and then the aqueous residue acidified to pH 1 with 1 M HCl. The precipitated carboxylic acid was filtered off, washed with water and dried. The carboxylic acid was reduced by adding CDI (1.5 equiv) to a suspension of carboxylic acid (1 equiv) in dry THF (10 mL) under an atmosphere of N2. After stirring for 18 h, the resulting solution was added dropwise to a solution of NaBH4 (5 equiv) in H2O (10 mL) and stirred for 30 min. The reaction was then quenched by the addition of 1 M HCl and stirred for a further 30 min. The solution was neutralised with saturated aqueous NaHCO3 and extracted twice with CH2Cl2. The combined organic layers were dried (Na2SO4) and the solvent removed in vacuo. Chromatography (eluting with a hexanes: EtOAc gradient) gave the alcohol. Reoxidation was carried out by stirring a suspension of the pyrazolo[1,5-a]pyridine-3-methanol (1 equiv) and MnO2 (10 equiv) in CH2Cl2 (2 mL) at room temperature for 4 days. The reaction mixture was then filtered through celite, washed with CH2Cl2, and the solvent removed from the filtrate in vacuo to leave the aldehyde.
References:
Kendall, Jackie D.;O'Connor, Patrick D.;Marshall, Andrew J.;Frédérick, Raphaël;Marshall, Elaine S.;Lill, Claire L.;Lee, Woo-Jeong;Kolekar, Sharada;Chao, Mindy;Malik, Alisha;Yu, Shuqiao;Chaussade, Claire;Buchanan, Christina;Rewcastle, Gordon W.;Baguley, Bruce C.;Flanagan, Jack U.;Jamieson, Stephen M.F.;Denny, William A.;Shepherd, Peter R. [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 1,p. 69 - 85] Location in patent:experimental part
![Pyrazolo[1,5-a]pyridine-3-carboxylic acid, 6-bromo-, methyl ester](/CAS/GIF/1062368-70-0.gif)
1062368-70-0
88 suppliers
$34.00/100mg
![6-BroMopyrazolo[1,5-a]pyridine](/CAS/GIF/1264193-11-4.gif)
1264193-11-4
121 suppliers
$89.00/250mg
626-55-1
573 suppliers
$5.00/5 / G
![6-BroMopyrazolo[1,5-a]pyridine](/CAS/GIF/1264193-11-4.gif)
1264193-11-4
121 suppliers
$89.00/250mg