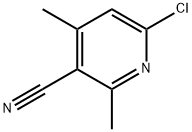
6-Chloro-2,4-dimethylnicotinonitrile synthesis
- Product Name:6-Chloro-2,4-dimethylnicotinonitrile
- CAS Number:101251-72-3
- Molecular formula:C8H7ClN2
- Molecular Weight:166.61

65934-19-2
4 suppliers
inquiry

101251-72-3
23 suppliers
inquiry
Yield:101251-72-3 70%
Reaction Conditions:
with N-benzyl-N,N,N-triethylammonium chloride;N,N-dimethyl-formamide;trichlorophosphate
Steps:
EXPERIMENTAL
Our study is briefly described by Scheme 1. As is well known, Vilsmeier reagent (DMF/POCl3)can be used to dehydrate an amide group, convertingit into a nitrile group [2]. We transformed the amidegroup of pyridone (1) into a nitrile group (2) using thisapproach. The reaction mixture was refluxed for 5 hyielding a dark brown preparation. The product wasisolated via extraction in a Soxhlet apparatus withchloroform. A light yellow product was obtained witha yield of 52%.The next step was to obtain chloropyridine (3).Pyridone (2) was added to a mixture of DMF withPOCl3 and ref luxed for 5 h. After purification viacolumn chromatography, a white crystalline solid(as needles) was obtained with a yield of 62%.A similar method was used to obtain chloropyridine(1) without transforming the side amide groupinto a nitrile group. The reaction was conducted infreshly distilled POCl3 in the presence of triethylbenzylammoniumchloride to increase the concentrationof Cl- ions. Chloropyridine (3), however,was obtained instead of expected reactionproduct (4). The yield was 70%, higher than in twostepsynthesis.
References:
Koval’, Ya. I.;Okul’;Yatsenko;Babaev;Polyakova;Rybakov [Russian Journal of Physical Chemistry,2017,vol. 91,# 2,p. 246 - 251][Zh. Fiz. Khim.,2017,vol. 91,# 2,p. 247 - 252,6]
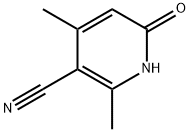
1704-19-4
13 suppliers
inquiry

101251-72-3
23 suppliers
inquiry