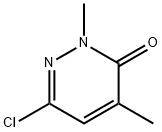
6-Chloro-2,4-dimethylpyridazin-3(2H)-one synthesis
- Product Name:6-Chloro-2,4-dimethylpyridazin-3(2H)-one
- CAS Number:1114563-58-4
- Molecular formula:C6H7ClN2O
- Molecular Weight:158.59
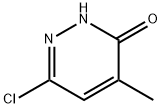
1834-27-1
73 suppliers
$18.00/100mg

74-88-4
357 suppliers
$15.00/10g

1114563-58-4
23 suppliers
inquiry
Yield: 82.87%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 20; for 17 h;
Steps:
1B.4 Step 4: Synthesis of 6-Chloro-2,4-dimethylpyridazin-3-one
To a stirred mixture of 6-chloro-4-methyl-2H-pyridazin-3- one (2.20 g, 15.22 mmol, 1.00 equiv) and K2CO3(4.21 g, 30.44 mmol, 2.00 equiv) in DMF (40.00 mL) was added Mel (2.38 g, 16.77 mmol, 1.10 equiv) dropwise at room temperature. The resulting mixture was stirred for 17 h at room temperature. The reaction was quenched with ice/water (120 mL) at 0 degrees C. The resulting mixture was extracted with EA (3x200 mL). The combined organic layers were washed with NaCl (3x200 mL), dried over anhydrous Na2S04. After filtration, the filtrate was concentrated under reduced pressure. 6-Chloro-2,4-dimethylpyridazin-3- one (2.00 g, 82.87%) was obtained as pink solid. LC/MS: mass calcd. For C6H7CIN2O: 158.02, found: 159.05 [M+H]+.
References:
DESIGN THERAPEUTICS, INC. WO2021/158707, 2021, A1 Location in patent:Paragraph 00616; 00668; 00675; 00676
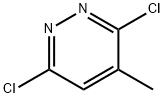
19064-64-3
271 suppliers
$6.00/5g

1114563-58-4
23 suppliers
inquiry