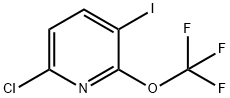
6-chloro-3-iodo-2-(trifluoroMethoxy)pyridine synthesis
- Product Name:6-chloro-3-iodo-2-(trifluoroMethoxy)pyridine
- CAS Number:1221171-95-4
- Molecular formula:C6H2ClF3INO
- Molecular Weight:323.44
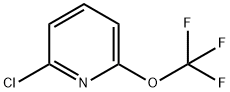
1221171-70-5
86 suppliers
$71.00/250mg

1221171-95-4
34 suppliers
$200.00/100mg
Yield:1221171-95-4 78%
Reaction Conditions:
Stage #1: 2-chloro-6-(trifluoromethoxy)pyridinewith n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at -78;
Stage #2: with iodine in tetrahydrofuran;hexane at 25;
Steps:
2
2-Chloro-5-iodo-6-trifluoromethoxy pyridine (41); At 0 0C, diisopropylamine (2.2 g, 3.1 mL, 22.2 mmol, 1.1 eq) was added dropwise to a solution of butyllithium (1.56 M in hexane, 14.2 mL, 22.2 mmol, 1.1 eq) in THF (35 mL). At -78 0C, a solution of 2-chloro-6-trifluoromethoxypyridine (2, 4.0 g, 20.2 mmol,1 eq) in THF (10 mL) was added dropwise followed after 2 h by a solution of iodide (5.7 g, 22.2 mmol, 1.1 eq) in THF (10 mL). The reaction mixture was allowed to reach 25 0C before being treated with a saturated aqueous solution of sodium sulfite (30 mL) and extracted with dichloromethane (3 x 20 mL). The combined organic layers were dried over sodium sulfate before being evaporated. The crude dark oil was distilled under vacuum (b.p. 103-106 0C / 16 mbar) to afford pure 2-chloro-5-iodo-6- trifluoromethoxypyridine (41, 5.1 g, 15.7 mmol, 78%) as colorless needles; m.p. 33-350C.1H NMR (CDCl3, 300 MHz): δ = 8.11 (d, J = 8.1 Hz, 1 H), 7.03 (d, J= 8.1 Hz, I H). - 19F NMR (CDCl3, 282 MHz): δ = -57.1 - 13C NMR (CDCl3, 75 MHz): δ = 154.9, 151.7, 148.8, 142.0, 123.3, 120.4 (q, J = 264 Hz).
References:
WO2010/40461,2010,A1 Location in patent:Page/Page column 43-44