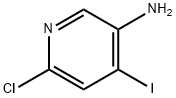
6-CHLORO-4-IODOPYRIDIN-3-AMINE synthesis
- Product Name:6-CHLORO-4-IODOPYRIDIN-3-AMINE
- CAS Number:351227-42-4
- Molecular formula:C5H4ClIN2
- Molecular Weight:254.46
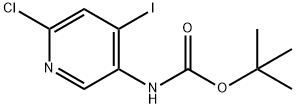
400777-00-6

351227-42-4
Step c: Synthesis of 6-chloro-4-iodo-pyridin-3-amine Tert-butyl (6-chloro-4-iodo-pyridin-3-yl)-carbamate (10.0 g, 28 mmol) was dissolved in 3 M hydrochloric acid (600 mL), heated to 60°C, and reacted for 12 hours. After completion of the reaction, the mixture was cooled to room temperature and the pH was adjusted with saturated sodium bicarbonate solution to 8. Subsequently, the aqueous layer was extracted with ethyl acetate (100 mL × 3). The organic layers were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography with the eluent being a petroleum ether solution of 10% ethyl acetate to give 6-chloro-4-iodopyridin-3-amine (6.6 g, 93% yield). 1H-NMR (CDCl3, 400 MHz) δ 7.81 (s, 1H), 7.60 (s, 1H), 4.13 (br s, 2H).

400777-00-6
95 suppliers
$55.00/1g

351227-42-4
104 suppliers
$52.00/100mg
Yield: 93%
Reaction Conditions:
Stage #1:(6-chloro-4-iodopyridin-3-yl)carbamic acid tert-butyl ester with hydrogenchloride;water at 60; for 12 h;
Stage #2: with sodium hydrogencarbonate in water; pH=8 at 20;Saturated solution;
Steps:
9.c
Step c: 6-chloro-4-iodopyridin-3-amine The solution of tert-butyl 6-chloro-4-iodopyridin-3-ylcarbamate (10.0 g, 28 mmol) in 3M HCl (600 mL) was heated at 60° C. for 12 h. The mixture was allowed to cool to room temperature and treated with sat. NaHCO3 to pH=8. The aqueous layer was extracted with ethyl acetate (100 mL*3). The combined organic layers were washed with brine, dried over anhydrous Na2SO4, concentrated and purified by chromatography on silica gel (10% ethyl acetate in petroleum ether as eluant) to afford 6-chloro-4-iodopyridin-3-amine (6.6 g, 93%). 1H-NMR (CDCl3, 400 MHz) δ 7.81 (s, 1H), 7.60 (s, 1H), 4.13 (br s, 2H).
References:
VERTEX PHARMACEUTICALS INCORPORATED US2009/253736, 2009, A1 Location in patent:Page/Page column 29
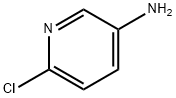
5350-93-6
428 suppliers
$6.00/5g

351227-42-4
104 suppliers
$52.00/100mg
![5-[N-(TERT-BUTOXYCARBONYL)AMINO]-2-CHLOROPYRIDINE](/CAS/GIF/171178-45-3.gif)
171178-45-3
144 suppliers
$8.00/250mg

351227-42-4
104 suppliers
$52.00/100mg