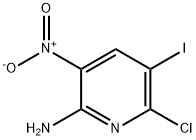
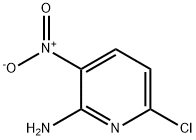
6-Chloro-5-iodo-3-nitropyridin-2-amine synthesis
- Product Name:6-Chloro-5-iodo-3-nitropyridin-2-amine
- CAS Number:790692-90-9
- Molecular formula:C5H3ClIN3O2
- Molecular Weight:299.45
27048-04-0

790692-90-9
The general procedure for the synthesis of 6-chloro-5-iodo-3-nitropyridin-2-amine from 2-amino-3-nitro-6-chloropyridine was as follows: to a solution of 6-chloro-3-nitropyridin-2-amine (630 mg, 3.63 mmol) in ethanol (11 mL) were added sequentially iodine (920 mg, 3.62 mmol) and silver sulfate (1132 mg, 3.63 mmol). The reaction mixture was stirred overnight at room temperature, then diluted with water (100 mL) and extracted with ethyl acetate (3 × 80 mL). The organic phases were combined, washed with brine (50 mL), dried over anhydrous sodium sulfate, and concentrated under reduced pressure to afford 6-chloro-5-iodo-3-nitropyridin-2-amine (640 mg, 59%) as a yellow solid. Subsequently, 6-chloro-5-iodo-3-nitropyridin-2-amine (640 mg, 2.14 mmol) was dissolved in a solvent mixture of ethanol (40 mL) and water (10 mL), to which iron powder (1.93 g, 34.46 mmol) and ammonium chloride (887 mg, 16.58 mmol) were added. The reaction mixture was heated to reflux for 4 h and then concentrated, and the residue was dissolved in water (100 mL) and extracted with ethyl acetate (3 × 80 mL). The organic phases were combined, washed with brine (50 mL), dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography to afford 6-chloro-5-iodopyridine-2,3-diamine (560 mg, 97%) as a brown solid, using a 33% petroleum ether solution of ethyl acetate as eluent. Next, a 6-chloro-5-iodopyridine-2,3-diamine (100 mg, 0.37 mmol), (2,3-dichlorophenyl)boronic acid (147.3 mg, 0.77 mmol), tetrakis(triphenylphosphine)palladium (42.9 mg, 0.04 mmol), and sodium carbonate (118.2 mg, 1.12 mmol) solution in water (5 mL) and dioxane (15 mL) solution was heated to reflux overnight. Upon completion of the reaction, it was quenched with water (100 mL) and extracted with ethyl acetate (3 × 50 mL). The organic phases were combined, washed with brine (50 mL), dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography to afford 6-chloro-5-(2,3-dichlorophenyl)pyridine-2,3-diamine (80 mg, 75%) as a brown solid, using a petroleum ether solution of 50% ethyl acetate as eluent. Finally, a solution of 6-chloro-5-(2,3-dichlorophenyl)pyridine-2,3-diamine (80 mg, 0.28 mmol) in trifluoroacetic acid (10 mL) and concentrated hydrochloric acid (2 mL) was heated to reflux overnight. The reaction mixture was quenched with water (100 mL), pH adjusted to 8 with sodium carbonate, and extracted with ethyl acetate (3 × 80 mL). The organic phases were combined, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography using a petroleum ether solution of 50% ethyl acetate as eluent to afford 5-chloro-6-(2,3-dichlorophenyl)-2-(trifluoromethyl)-1H-imidazo[4,5-b]pyridine trifluoroacetate (2 mg, 2%) as an off-white solid.

27048-04-0
349 suppliers
$5.00/250mg

790692-90-9
36 suppliers
$91.00/50mg
Yield:790692-90-9 88%
Reaction Conditions:
with iodine;silver sulfate in ethanol at 20;
Steps:
5.a
To a suspension of 6-chloro-3-nitropyridin-2-amine (6.3 g, 36.3 mmol)in ethanol (110 ml_), 9.2 g (36.3 mmol) of iodine and 1 1.32 g (36.3 mmol) of silver sulphate were added. The crude mixture was stirred at room temperature overnight and the precipitate formed was filtered off. The solid isolated was purified by flash chromatography (1 :1 hexane/ethyl acetate) to give the title compound (9.74 g, 88% of yield). δ 1H-NMR (CDCI3): 1.56 (s, 2H), 8.76 (s, 1H).ESI/MS m/e: 300 ([IvH-H]+, C5H3CIIN3O2)
References:
WO2007/39297,2007,A1 Location in patent:Page/Page column 28