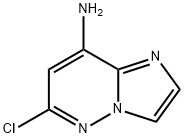
6-Chloro-imidazo[1,2-b]pyridazin-8-ylamine synthesis
- Product Name:6-Chloro-imidazo[1,2-b]pyridazin-8-ylamine
- CAS Number:1161847-36-4
- Molecular formula:C6H5ClN4
- Molecular Weight:168.58
![Tert-butyl N-(6-chloroimidazo[1,2-b]pyridazin-8-yl)carbamate](/CAS/20210305/GIF/1161847-35-3.gif)
1161847-35-3
0 suppliers
inquiry
![6-Chloro-imidazo[1,2-b]pyridazin-8-ylamine](/CAS/20180703/GIF/1161847-36-4.gif)
1161847-36-4
27 suppliers
inquiry
Yield:1161847-36-4 100%
Reaction Conditions:
Stage #1: (6-chloroimidazo[1,2-b]pyridazin-8-yl)carbamic acid tert-butyl esterwith trifluoroacetic acid in dichloromethane; for 5 h;
Stage #2: with sodium hydrogencarbonate in dichloromethane;water;
Steps:
12
Example 12.; A solution of (6-chloro-imidazo[l,2-b]pyridazin-8-yl)-carbamic acid tert-butyl ester (1.59 g, 5.91 mmol) in 30 mL of dichloromethane and 15 mL of trifluoro acetic acid was stirred for 5 h then concentrated to a yellow oil, which was treated (slowly) with 50 mL of a saturated aq. NaHCOs solution. Undissolved solid was isolated by Buchner filtration, rinsing well with water and dried by sucking air through then in vacuo to afford 1.10 g (102% crude) of 6-chloro-imidazo[l,2-b]pyridazin-8-ylamine as a yellow solid that was used without further purification.
References:
WO2009/77334,2009,A1 Location in patent:Page/Page column 85