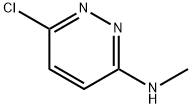
6-Chloro-N-methylpyridazin-3-amine synthesis
- Product Name:6-Chloro-N-methylpyridazin-3-amine
- CAS Number:14959-32-1
- Molecular formula:C5H6ClN3
- Molecular Weight:143.57
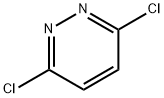
141-30-0

74-89-5

14959-32-1
3,6-Dichloropyridazine (107,500 mg, 3.36 mmol) was slowly added to 2.0 M methanol solution of methylamine (MeOH solution of NH2Me, 5 mL, 10 mmol) and stirred at room temperature for 24 hours. Upon completion of the reaction, water was added to the reaction mixture and the organic phase was extracted with ethyl acetate. The collected organic phase was washed with saturated aqueous sodium chloride solution, dried over anhydrous sodium sulfate and concentrated under reduced pressure. Purification by column chromatography afforded 3-chloro-6-methylaminopyridazine (3-(N-monomethylamino)-6-chloropyridazine, 108e, 505 mg, 88% yield) as a white solid.1H NMR (400 MHz, DMSO-d6) δ 2.84 (d, J = 4.4 Hz, 3H), 6.87 (d, J = 9.2 Hz, 1H), 7.06 (br d, J = 4.0 Hz, 1H), 7.33 (d, J = 9.2 Hz, 1H); 13C NMR (100 MHz, DMSO-d6) δ 27.9, 117.9, 128.2, 144.9, 158.6.
Yield:14959-32-1 88%
Reaction Conditions:
in methanol at 20; for 24 h;
Steps:
29
3,6-Dichloropyridazine (107, 500 mg, 3.36 mmol) was slowly added to a 2.0 M methylamine methanol solution (NH2Me in MeOH, 5 mL, 10 mmol) and stirred at room temperature for 24 hours. Water was added to the reaction mixture, and organic compounds were extracted with ethyl acetate. The recovered organic solution was washed with an aqueous solution of saturated sodium chloride and evaporated after a treatment with sodium sulfate. Purification was performed by column chromatograph to give the target compound 3-(N-monomethylamino)-6-chloropyridazine (108e, 505 mg, 88%) as a white solid.1H NMR (400 MHz, DMSO-d6) δ 2.84 (d, J=4.4 Hz, 3H), 6.87 (d, J=9.2 Hz, 1H), 7.06 (brd, J=4.0 Hz, 1H), 7.33 (d, J=9.2 Hz, 1H); 13C NMR (100 MHz, DMSO-d6) δ 27.9, 117.9, 128.2, 144.9, 158.6.
References:
US2010/261727,2010,A1 Location in patent:Page/Page column 32

141-30-0
402 suppliers
$6.00/10g

14959-32-1
82 suppliers
$10.00/1g

15209-31-1
0 suppliers
inquiry

14959-32-1
82 suppliers
$10.00/1g