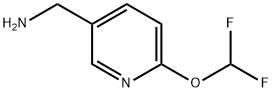
(6-(difluoroMethoxy)pyridin-3-yl)MethanaMine synthesis
- Product Name:(6-(difluoroMethoxy)pyridin-3-yl)MethanaMine
- CAS Number:1198103-43-3
- Molecular formula:C7H8F2N2O
- Molecular Weight:174.15

1198103-42-2

1198103-43-3
General procedure for the synthesis of 6-(difluoromethoxy)pyridine-3-carbonitrile from 6-(difluoromethoxy)pyridine-3-methanamine: Raney nickel (150 mg) was added to a solution of 6-(difluoromethoxy)nicotinonitrile (0.541 g, 3.18 mmol) in methanol (20 mL, saturated with ammonia). The reaction mixture was hydrogenated at room temperature under 45 psi hydrogen pressure for 20 hours. Upon completion of the reaction, the reaction mixture was filtered through a short bed of diatomaceous earth and the filtrate was concentrated under vacuum. The crude product was purified by silica gel column chromatography using dichloromethane:methanol=90:10 as eluent to afford 6-difluoromethoxypyridine-3-methanamine as a light yellow oil in a yield of 440 mg (80% yield). The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3): δ 8.12 (d, 1H), 7.73 (dd, 1H), 7.45 (t, 1H), 6.88 (d, 1H), 3.88 (s, 2H) (NH2 not shown); 19F NMR (400 MHz, CDCl3) δ -88.98 and -89.17; MS ( ESI) m/z 175 [M + H]+.

1198103-42-2
18 suppliers
inquiry

1198103-43-3
38 suppliers
$45.00/10mg
Yield:1198103-43-3 80%
Reaction Conditions:
with hydrogen;nickel in methanol at 16 - 25; under 2327.23 Torr; for 20 h;
Steps:
I-10
Raney/Nickel (150 mg) was added to a solution of 6-(difluoromethoxy)nicotinonitrile(0.541 g, 3.18 mmol) in methanol (20 mL, saturated with ammonia). The mixture was hydrogenated (45 psi) at room temperature for 20 hours then filtered through a short bed of celite and concentrated in vacuo. The crude amine was purified by silica gel column chromatography using dichloromethane : methanol = 90:10 as eluent giving the title compound as pale yellow oil, 440 mg (80 %).1H NMR (400 MHz, CDCl3) δ (ppm) 8.12 (d, IH) 7.73 (dd, 1 H) 7.45 (t, 1 H) 6.88 (d, 1 H) 3.88 (s, 2 H) (NH2 not shown). 19F NMR (400 MHz, CDCl3) δ (ppm) -88.98 and -89.17.MS (ESI) m/z 175 [M+H].
References:
WO2009/145720,2009,A1 Location in patent:Page/Page column 29