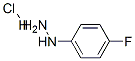6-Fluoro-1H,2H,3H,4H,9H-pyrido[3,4-b]indole synthesis
- Product Name:6-Fluoro-1H,2H,3H,4H,9H-pyrido[3,4-b]indole
- CAS Number:17952-80-6
- Molecular formula:C11H11FN2
- Molecular Weight:190.22
Yield:17952-80-6 54%
Reaction Conditions:
with sulfuric acid in 1,4-dioxane; for 3 h;
Steps:
1.A Step A
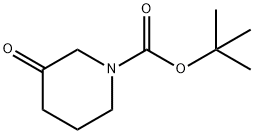
To a solution of (4-fluorophenyl)hydrazine HCI-salt (1 g, 6.1 mmol) and tert-butyl 3- oxopiperidine-1 -carboxylate (1.2 g, 6.1 mmol) in 1 ,4-dioxane (10 mL) was added cone. H2SO4 (1 mL), 0 °C. Then the reaction mixture was heated at 1 10 °C for 3 h. The reaction mixture was cooled to 25 °C and the precipitate was filtered off. The solid was dissolved in water basified with NaOH solution and extracted with dichloromethane. The organic phase was separated, dried over Na2S04, filtered and the solvent was removed under reduced pressure to afford the cyclized title compound as a pale yellow solid (0.6 g, 54 %). (0212) 1 H-NMR (400 MHz, DMSO-ck) d = 10.73 (br-s, 1 H), 7.21-7.22 (m, 1 H), 7.06-7.07 (m, 1 H), 6.78- 6.79 (m, 1 H), 3.83 (s, 2H), 2.94-2.95 (m, 2H), 2.49-2.50 (m, 2H). (0213) MS: 191 (M+H)+.
References:
WO2019/233883,2019,A1 Location in patent:Page/Page column 43-44
![6-fluoro-1H,2H,3H,4H,9H-pyrido[3,4-b]indol-1-one](/CAS/20180713/GIF/778-73-4.gif)
778-73-4
3 suppliers
inquiry
![6-Fluoro-1H,2H,3H,4H,9H-pyrido[3,4-b]indole](/CAS/20180529/GIF/17952-80-6.gif)
17952-80-6
17 suppliers
inquiry