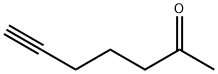
6-Heptyn-2-one (7CI,8CI,9CI) synthesis
- Product Name:6-Heptyn-2-one (7CI,8CI,9CI)
- CAS Number:928-39-2
- Molecular formula:C7H10O
- Molecular Weight:110.15
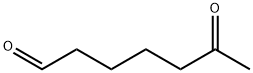
19480-04-7
0 suppliers
inquiry

928-39-2
7 suppliers
inquiry
Yield:928-39-2 76%
Reaction Conditions:
with Nonafluorobutanesulfonyl fluoride;tert-butylimino-tri(pyrrolidino)phosphorane in N,N-dimethyl-formamide at -30 - 20;Inert atmosphere;chemoselective reaction;
Steps:
4.4. Synthesis of alkynyl ketones 2
4.4.1. General procedure (GP1).Starting keto aldehyde 1 (1.00 mmol), NfF (0.305 g, 1.01 mmol) and DMF (1 mL) were placed in a round-bottom flask. The flask was cooled to -30 °C whereupon the P1-base (0.628 g, 2.01 mmol) was added dropwise. The reaction mixture was allowed to warm up gradually to rt and stirred for 16-18 h (NMR monitoring). It was quenched with saturated aq NH4Cl (15 mL) and extracted with Et2O (3×15 mL). The combined organic layers were washed with water (15 mL), brine (15 mL), dried over MgSO4, and concentrated. Products 2 were isolated by column chromatography on SiO2.
References:
Boltukhina, Ekaterina V.;Sheshenev, Andrey E.;Lyapkalo, Ilya M. [Tetrahedron,2011,vol. 67,# 30,p. 5382 - 5388] Location in patent:experimental part
![6-methyl-7-oxabicyclo[4.1.0]heptan-2-one](/CAS/GIF/21889-89-4.gif)
21889-89-4
18 suppliers
$160.00/250mg

928-39-2
7 suppliers
inquiry
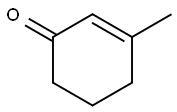
1193-18-6
173 suppliers
$6.00/1g

928-39-2
7 suppliers
inquiry
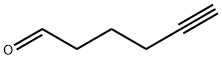
29329-03-1
11 suppliers
inquiry

928-39-2
7 suppliers
inquiry
928-90-5
194 suppliers
$8.00/250mg

928-39-2
7 suppliers
inquiry