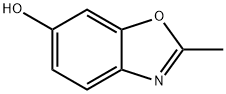
6-Hydroxy-2-methylbenzoxazole synthesis
- Product Name:6-Hydroxy-2-methylbenzoxazole
- CAS Number:5078-07-9
- Molecular formula:C8H7NO2
- Molecular Weight:149.15

111364-29-5

5078-07-9
The general procedure for the synthesis of 2-methyl-6-hydroxybenzoxazole from the compound (CAS:111364-29-5) was as follows: (1Z)-1-(2,4-dihydroxyphenyl)ethanone oxime (9.7 g, 57.7 mmol) was dissolved in a mixed solvent of acetonitrile (65 mL) and dimethylacetamide (11 mL) and cooled to 5 °C. Phosphorus trichloride (5.6 mL, 60.3 mmol) was slowly added dropwise at a temperature not exceeding 10 °C. After the dropwise addition, the reaction mixture was stirred at room temperature for 1 h. The reaction solution gradually formed a yellow slurry. The reaction mixture was carefully poured into a pre-prepared mixture of sodium bicarbonate and ice for neutralization. The resulting precipitate was collected by filtration and dried to give 6.3 g of 2-methyl-6-hydroxybenzoxazole in 73% yield.
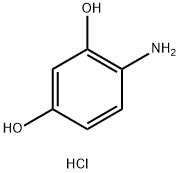
34781-86-7
109 suppliers
$69.00/1 / G

75-36-5
589 suppliers
$17.92/100G

5078-07-9
60 suppliers
$60.00/250mg
Yield:-
Reaction Conditions:
with pyridinium p-toluenesulfonate;triethylamine in ethyl acetate
Steps:
34 2-Methyl-benzoxazol-6-ol
PREPARATION 34 2-Methyl-benzoxazol-6-ol 4-Aminoresorcinol hydrochloride (2.0 g, 12.4 mM), acetyl chloride (1.0 g, 12.6 mM), triethylamine (1.38 g, 13.6 mM) and pyridinium-p-toluenesulfonate (PPTS, 800 mg, 3.2 mM) were refluxed in xylenes (50 mL) for about 18 hours. Additional PPTS (300 mg) was added and the mixture was then refluxed about 48 hours. The reaction mixture was concentrated and the residue dissolved in ethyl acetate (200 mL) and washed with H2 O (3*150 mL). The combined aqueous layer was back extracted with ethyl acetate (200 mL) and the combined organic layers were dried over MgSO4. Filtration and concentration provided 1.36 g. Filtration through a silica gel column eluted with 10% methanol/methylene chloride provided an orange solid, 0.3 g; m.p., 94-96° C.
References:
Pfizer Inc US6130217, 2000, A
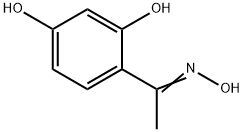
6134-79-8
36 suppliers
$57.50/250 mg

5078-07-9
60 suppliers
$60.00/250mg

111364-29-5
0 suppliers
inquiry

5078-07-9
60 suppliers
$60.00/250mg
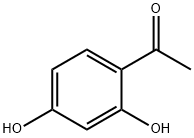
89-84-9
569 suppliers
$6.00/10g

5078-07-9
60 suppliers
$60.00/250mg

89-84-9
569 suppliers
$6.00/10g

6134-79-8
36 suppliers
$57.50/250 mg

5078-07-9
60 suppliers
$60.00/250mg