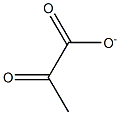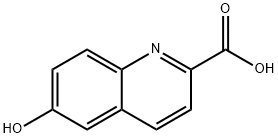
6-Hydroxy-2-quinolinecarboxylic acid synthesis
- Product Name:6-Hydroxy-2-quinolinecarboxylic acid
- CAS Number:75434-18-3
- Molecular formula:C10H7NO3
- Molecular Weight:189.17
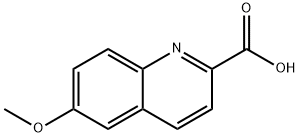
75433-99-7

75434-18-3
6-Methoxyquinoline-2-carboxylic acid (4 g, 0.019 mol) was used as starting material and suspended in 48% aqueous hydrobromic acid (80 ml). The reaction mixture was heated to reflux at 125°C overnight. Upon completion of the reaction, the mixture was cooled to 0 °C and the pH was adjusted to 6-7 by slow addition of aqueous ammonium hydroxide. subsequently, dilute hydrochloric acid was added dropwise until the pH reached 3-4, at which point the target product, 6-hydroxy-2-quinolinecarboxylic acid, precipitated as a solid. The precipitate was collected by filtration, washed thoroughly with deionized water, and finally dried in a vacuum drying oven to give 3.5 g of the product in the form of a yellow solid (0.0185 mol, 97% yield). The molecular weight of the product was confirmed to be 190.1 (M+H)+ by mass spectrometry.

429687-75-2
28 suppliers
$438.50/250mg

75434-18-3
35 suppliers
$140.00/50mg
Yield:75434-18-3 99%
Reaction Conditions:
with lithium hydroxide monohydrate in tetrahydrofuran;lithium hydroxide monohydrate at 20; for 0.5 h;
Steps:
Step 3.
Lithium hydroxide (3.61 g, 151 mmol) was added to a mixtureof methyl 6-hydroxyquinoline-2-carboxylate 41 (10.2 g, 50.2 mmol),THF (200 mL) and water (70 mL) at ambient temperature. After 30 min,the THF was removed under reduced pressure, and the reaction mixturewas acidified with 1 M HCl to pH ~ 1. The precipitate was collected byfiltration and washed with water. The solid was dried for 18 h undervacuum at 50 C to give 6-hydroxyquinoline-2-carboxylic acid, 42 (9.40g, 99% yield).
References:
Patel, Meena V.;Peltier, Hillary M.;Matulenko, Mark A.;Koenig, John R.;C. Scanio, Marc J.;Gum, Rebecca J.;El-Kouhen, Odile F.;Fricano, Meagan M.;Lundgaard, Greta L.;Neelands, Torben;Zhang, Xu-Feng;Zhan, Cenchen;Pai, Madhavi;Ghoreishi-Haack, Nayereh;Hudzik, Thomas;Gintant, Gary;Martin, Ruth;McGaraughty, Steve;Xu, Jun;Bow, Daniel;Kalvass, John C.;Kym, Philip R.;DeGoey, David A.;Kort, Michael E. [Bioorganic and Medicinal Chemistry,2022,vol. 63,art. no. 116743]

75433-99-7
50 suppliers
$150.00/250mg

75434-18-3
35 suppliers
$140.00/50mg

52313-34-5
14 suppliers
inquiry

75434-18-3
35 suppliers
$140.00/50mg
42454-06-8
215 suppliers
$8.00/1g

75434-18-3
35 suppliers
$140.00/50mg