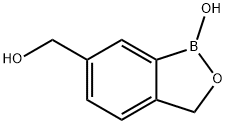
6-(hydroxymethyl)benzo[c][1,2]oxaborol-1(3H)-ol synthesis
- Product Name:6-(hydroxymethyl)benzo[c][1,2]oxaborol-1(3H)-ol
- CAS Number:947162-87-0
- Molecular formula:C8H9BO3
- Molecular Weight:163.97
Yield:947162-87-0 77%
Reaction Conditions:
Stage #1: 2,2’-(2-bromo-1,4-phenylene)bis(methylene)bis(oxy)bis(tetrahydro-2H-pyran)with n-butyllithium in tetrahydrofuran;hexane at -78; for 0.5 h;Inert atmosphere;
Stage #2: Triisopropyl borate in tetrahydrofuran;hexane at -78 - 20; for 3 h;Inert atmosphere;
Stage #3: with toluene-4-sulfonic acid in methanol at 20; for 5 h;Inert atmosphere;
Steps:
Synthesis of 5-hydroxymethylbenzoxaborole (4c)
To a flame-dried round-bottom flask was added S6 (5.2 g, 13.5 mmol) and THF (50 mL). The flask was cooled to -78 C and BuLi solution (2.5 M in hexanes, 5.6 mL, 14 mmol) was added dropwise via syringe over 30 minutes. After the addition, the mixture was stirred at -78 C for 30 minutes. B(OiPr)3 (3.2 mL, 15.1 mmol) was added via syringe at -78 C and the reaction was warmed to room temperature to stir for 3 hours. Then, the reaction was cooled to 0C, quenched with saturated NH4Cl (40 mL), and diluted with water/EtOAc. The aqueous layer was extracted with EtOAc (3 x 50 mL) and combined organics were washed with brine, dried over Na2SO4, and concentrated. The crude residue was dissolved in MeOH (100 mL) and p-TSA (1.39 g, 7.30 mmol) was added. The mixture was stirred for 5 hours at room temperature and then concentrated to an oil. The residue was quickly dissolved in EtOAc and extracted (3 x 50 mL) against water (~40 mL). Combined organics were washed with brine and dried over Na2SO4. Solvent removal produced an off-white solid, which was suspended in hexanes, filtered, and washed with diethyl ether (3 x 20 mL) to produce a white solid 4c (1.7 g, 77%). The compound was analytically pure and did not require further purification.1H NMR (MeOD, 400 MHz) δ 4.52 (s, 2H), 4.95 (s, 2H), 7.34 (d, 1H, J=7.8), 7.40 (d, 1H, J=7.8), 7.38 (s, 1H).11B NMR (MeOD, 128 MHz) δ 32.01.
References:
WO2019/173814,2019,A1 Location in patent:Page/Page column 33; 37; 38

5419-55-6
386 suppliers
$12.00/5g

7732-18-5
497 suppliers
$13.50/100ML
![2H-Pyran, 2,2'-[(2-bromo-1,4-phenylene)bis(methyleneoxy)]bis[tetrahydro-](/CAS/20210305/GIF/403699-41-2.gif)
403699-41-2
0 suppliers
inquiry
![6-(hydroxymethyl)benzo[c][1,2]oxaborol-1(3H)-ol](/CAS/20180703/GIF/947162-87-0.gif)
947162-87-0
7 suppliers
inquiry
![2H-Pyran, 2,2'-[(2-bromo-1,4-phenylene)bis(methyleneoxy)]bis[tetrahydro-](/CAS/20210305/GIF/403699-41-2.gif)
403699-41-2
0 suppliers
inquiry
![6-(hydroxymethyl)benzo[c][1,2]oxaborol-1(3H)-ol](/CAS/20180703/GIF/947162-87-0.gif)
947162-87-0
7 suppliers
inquiry

1393477-21-8
0 suppliers
inquiry
![6-(hydroxymethyl)benzo[c][1,2]oxaborol-1(3H)-ol](/CAS/20180703/GIF/947162-87-0.gif)
947162-87-0
7 suppliers
inquiry
586-35-6
203 suppliers
$11.00/5g
![6-(hydroxymethyl)benzo[c][1,2]oxaborol-1(3H)-ol](/CAS/20180703/GIF/947162-87-0.gif)
947162-87-0
7 suppliers
inquiry