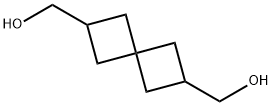
(6-Hydroxymethyl-spiro[3.3]hept-2-yl)-methanol synthesis
- Product Name:(6-Hydroxymethyl-spiro[3.3]hept-2-yl)-methanol
- CAS Number:138515-21-6
- Molecular formula:C9H16O2
- Molecular Weight:156.22
![Spiro[3.3]heptane-2,6-dicarboxylic acid, diethyl ester, (R)- (9CI)](/CAS/20210305/GIF/142292-56-6.gif)
142292-56-6
0 suppliers
inquiry
![(6-Hydroxymethyl-spiro[3.3]hept-2-yl)-methanol](/CAS/20180527/GIF/138515-21-6.gif)
138515-21-6
6 suppliers
inquiry
Yield:138515-21-6 88%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at -78 - 20; for 0.666667 h;
Steps:
29.29A Step 29 A. Spiro[3,3] heptane-2,6-dimethanol
To a cooled solution of diethyl spiro[3.3]heptane-2,6-dicarboxylate (5g, 20.81 mmol) in THF (60 mL) at -78 °C was added 1M LAH in THF (41.6 mL, 41.6 mmol) dropwise over 10 min period. After addition, the cold bath was removed and the resulting solution was allowed to warm to RT and kept at RT for 30 min. The reaction was cooled to 0 °C and quenched by addition of EtO Ac (100 mL), followed by IN HC1 (50 mL). The layers were separated and the aqueous layer was extracted with EtO Ac (4 x 100 mL). The combined organic layers were washed with brine, dried over Na2S04, filtered, and concentrated. The resulting residue was purified by flash chromatography (40g silica gel cartridge, eluting with 0-100% EtO Ac/Hex) to give spiro[3.3]heptane-2,6-diyldimethanol (2870 mg, 18.37 mmol, 88 % yield) as a colorless oil. MS (ESI) m/z: 313.2 (2M+H)+; NMR (500 MHz, chloroform-d) δ 3.56 (d, J=6.9 Hz, 4H), 2.37 (td, J=7.5, 14.9 Hz, 2H), 2.15 (ddd, J=3.3, 8.0, 11.3 Hz, 2H), 2.01 (ddd, J=3.3, 8.0, 11.5 Hz, 2H), 1.78 (dd, J=7.4, 11.3 Hz, 2H), 1.70 (dd, J=7.6, 11.4 Hz, 2H), 1.23 (br s, 2H).
References:
WO2019/89665,2019,A1 Location in patent:Page/Page column 87
![Spiro[3.3]heptane-2,6-dicarboxylic acid, 2,6-dimethyl ester](/CAS/20180527/GIF/37942-79-3.gif)
37942-79-3
28 suppliers
inquiry
![(6-Hydroxymethyl-spiro[3.3]hept-2-yl)-methanol](/CAS/20180527/GIF/138515-21-6.gif)
138515-21-6
6 suppliers
inquiry
![Spiro[3.3]heptane-2,6-dicarbonyl dichloride, (2R)- (9CI)](/CAS/20210305/GIF/60334-24-9.gif)
60334-24-9
0 suppliers
inquiry
![(6-Hydroxymethyl-spiro[3.3]hept-2-yl)-methanol](/CAS/20180527/GIF/138515-21-6.gif)
138515-21-6
6 suppliers
inquiry