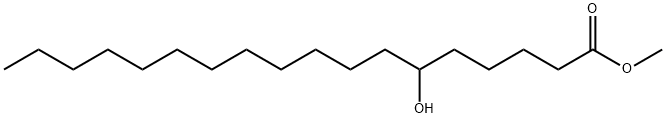
6-Hydroxyoctadecanoic acid methyl ester synthesis
- Product Name:6-Hydroxyoctadecanoic acid methyl ester
- CAS Number:2379-94-4
- Molecular formula:C19H38O3
- Molecular Weight:314.5
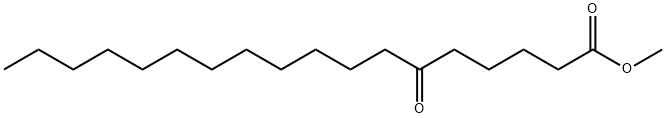
2380-21-4
1 suppliers
inquiry

2379-94-4
1 suppliers
inquiry
Yield:2379-94-4 94%
Reaction Conditions:
with sodium tetrahydroborate
Steps:
1 Preparation of n-hydroxystearate esters 10b-d
General procedure: n-Ketoesters 9b-d were previously described by Afri et al. (2014a).NaBH4 reduction of the latter, as described by Menger et al. (1989),yielded the corresponding hydroxyesters 10b-d. The products in eachcase were conveniently identified by their spectral data. The 13C NMRspectral data for 10b-d have been previously reported by Tulloch(1978) - though our chemical shifts and assignments are more completeand somewhat different. 2.3.1.1. Methyl 6-hydroxyoctadecanoate (10b). 94% yield, mp 48 °C; 1HNMR (CDCl3) δ 3.69 (s, 3H, OCH3), 3.62 (s, 1H, H6), 2.35 (t, J=7.2 Hz,2H, H2), 1.68 (m, 2H, H3), 1.45 (m, 8H, H4-H5 and H7-H8), 1.28 (m,18H, H9-H17, methylenes), 0.90 (”t”, J=7 Hz, 3H, H18). 13C NMR(CDCl3) δ 174.73 (C1), 71.75 (C6), 51.79 (OCH3), 37.36 and 37.00 (C5and C7), 34.62 (C2), 32.47 (C16), 30.12, 29.66 and 29.37 (C9-C15,methylenes), 25.65, 25.46 and 25.21 (C3, C4 and C8), 22.70 (C17), 14.13(C18). MS (DCI, CH4) m/z 297.282 (MH+-H2O, 100%); anal. HRMS (CI):calcd (C19H39O3, MH+) 297.2794 obsd. 297.2818. FTIR 3416 (vbr s,OH), 2926 (s), 2856 (m), 1747 (s, C]O), 1439 (w), 1199 (m, CeO)cm-1.
References:
Alexenberg, Carmit;Afri, Michal;Eliyahu, Shlomi;Porat, Hani;Ranz, Ayala;Frimer, Aryeh A. [Chemistry and Physics of Lipids,2019,vol. 221,p. 128 - 139]
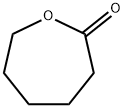
502-44-3
367 suppliers
$5.00/25g

2379-94-4
1 suppliers
inquiry
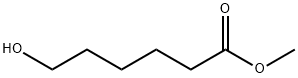
4547-43-7
80 suppliers
$19.00/100mg

2379-94-4
1 suppliers
inquiry
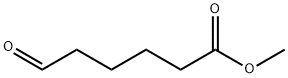
6654-36-0
91 suppliers
$20.00/250mg

15890-72-9
47 suppliers
$100.00/100ml

2379-94-4
1 suppliers
inquiry
