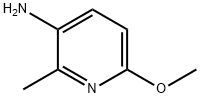
6-Methoxy-2-methylpyridin-3-amine synthesis
- Product Name:6-Methoxy-2-methylpyridin-3-amine
- CAS Number:52090-56-9
- Molecular formula:C7H10N2O
- Molecular Weight:138.17
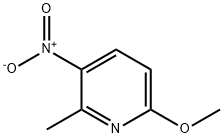
5467-69-6

52090-56-9
6-Methoxy-2-methyl-3-nitropyridine (2 g, 0.012 mol) was used as starting material, which was dissolved in H2O/MeOH (1:1, 40 mL) mixed solvent. Subsequently, NH4Cl (2.2 g, 3.5 eq.) and iron powder (2.3 g, 3.5 eq.) were added to the solution. The reaction mixture was stirred at 80 °C for 16 hours. After completion of the reaction, the iron powder was removed by filtration and the filtrate was washed with MeOH. The MeOH was removed from the filtrate by evaporation, diluted with H2O, and then the aqueous phase was extracted with EtOAc (3 x 50 mL). The organic phases were combined and dried with anhydrous Na2SO4. The desiccant was removed by filtration and the solvent was evaporated to dryness to give 2-methoxy-5-amino-6-methylpyridine (1.35 g, 81% yield) as a brown oil. Its nuclear magnetic resonance hydrogen spectrum (1H NMR, CDCl3) data were as follows: δ 6.9 (d, 1H), 6.4 (d, 1H), 3.8 (s, 3H), 2.33 (s, 3H).

5467-69-6
162 suppliers
$5.00/250mg

52090-56-9
132 suppliers
$16.00/250mg
Yield: 81%
Reaction Conditions:
with iron;ammonium chloride in methanol;water at 80; for 16 h;
Steps:
To a solution of intermediate 74 (2 g, 0.012 mol) in H20/MEOH 1: 1 (40 mL), were added NH4CI (2.2 g, 3.5 eq. ) and Fe powder (2.3 g, 3.5 eq. ). The reaction mixture was stirred at 80°C for 16 hr. Fe was then filtered and washed with MeOH. The MeOH was evaporated, H20 was added and the aqueous solution was extracted with EtOAc (3X50 mL). The combined organic extracts were dried over anh. NA2SO4, the solids were filtered and the solvent evaporated to dryness to give the title compound (1.35 g, 81 %) as a brown oil. NMR ('H, CDCI3) : 8 6.9 (d, 1 H), 6.4 (d, 1 H), 3.8 (s, 3H), 2.33 (s, 3H).
References:
SB PHARMCO PUERTO RICO INC;NEUROCRINE BIOSCIENCES INC;GLAXO GROUP LIMITED WO2004/62665, 2004, A1 Location in patent:Page/Page column 45