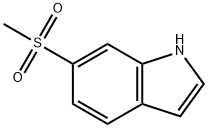
6-(METHYLSULFONYL)-1H-INDOLE synthesis
- Product Name:6-(METHYLSULFONYL)-1H-INDOLE
- CAS Number:467461-40-1
- Molecular formula:C9H9NO2S
- Molecular Weight:195.24
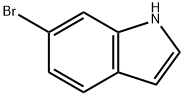
52415-29-9
417 suppliers
$9.00/1g
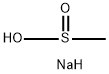
20277-69-4
282 suppliers
$10.00/1g

467461-40-1
23 suppliers
$113.00/250mg
Yield:467461-40-1 700 mg
Reaction Conditions:
with copper(l) iodide;L-proline in dimethyl sulfoxide at 80; for 12 h;Inert atmosphere;
Steps:
42.1 [312] Step 1: 6-methylsulfonyl-1H-indole
A mixture of 6-bromo-1H-indole (2.00 g, 10.20 mmol, 1.00 eq), BLAHsodium (1.35 g, 13.26 mmol, 1.30 eq), CuI (388.59 mg, 2.04 mmol, 0.20 eq) and L-PROLINE (469.82 mg, 4.08 mmol, 0.40 eq) in DMSO (20.00 mL) was degassed and purged with N2 for 3 times, and then the mixture was stirred at 80 C for 12 h under N2 atmosphere. The reaction mixture was quenched by addition saturated aqueous NH4Cl 200 mL at 20 C, and then extracted with EtOAc (100 mLx3). The combined organic layers were washed with brine (300 mLx2), dried over Na2SO4, filtered and concentrated under reduced pressure to give a residue. The residue was purified by column chromatography (SiO2, PE/EtOAc=10:1 to 1:1) to afford the title compound (700 mg) as an off-white solid.
References:
WO2018/13867,2018,A1 Location in patent:Paragraph 312