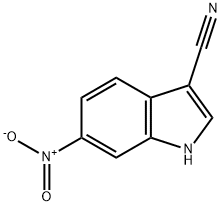
6-Nitro-1H-indole-3-carbonitrile synthesis
- Product Name:6-Nitro-1H-indole-3-carbonitrile
- CAS Number:4769-99-7
- Molecular formula:C9H5N3O2
- Molecular Weight:187.15
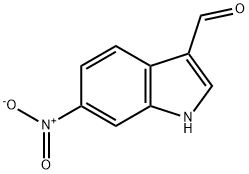
10553-13-6
44 suppliers
$110.00/100mg

4769-99-7
30 suppliers
inquiry
Yield:4769-99-7 88.1%
Reaction Conditions:
with hydroxylamine hydrochloride;sodium formate in 1-methyl-pyrrolidin-2-one;formic acid at 105; for 3 h;
Steps:
4.1.3. Synthesis of 5-nitro-1H-indole-3-carbonitrile (5)
General procedure: A mixture of compound 3 (1.5 g, 7.9 mmol), hydroxylaminehydrochloride (1.1 g,16 mmol), sodium methoxide (2.2 g, 32 mmol),formic acid (20 mL), and NMP (2 mL) was refluxed at 105 for 3 h.After the reaction was completed, the mixture was poured intowater at room temperature, stirred for 30 min, filtered, washedwith water, and dried to give compound 5 as a yellow solid (1.1 g,74.8%).
References:
Zhang, Bing;Duan, Yulin;Yang, Yuwei;Mao, Qing;Lin, Fengwei;Gao, Jun;Dai, Xiwen;Zhang, Peng;Li, Qiuhua;Li, Jinxin;Dai, Ronghua;Wang, Shaojie [European Journal of Medicinal Chemistry,2022,vol. 227,art. no. 113928]
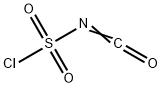
1189-71-5
365 suppliers
$18.00/5g
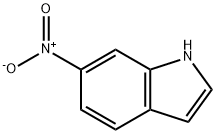
4769-96-4
266 suppliers
$9.00/250mg

4769-99-7
30 suppliers
inquiry

4769-96-4
266 suppliers
$9.00/250mg

4769-99-7
30 suppliers
inquiry