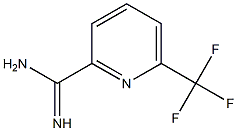
6-(Trifluoromethyl)picolinimidamide synthesis
- Product Name:6-(Trifluoromethyl)picolinimidamide
- CAS Number:790195-74-3
- Molecular formula:C7H6F3N3
- Molecular Weight:189.1378
Yield:790195-74-3 53%
Reaction Conditions:
with ammonium chloride in hydrogenchloride;diethyl ether;chloroform;toluene;
Steps:
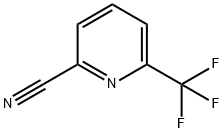
6-Trifluoromethyl-pyridine-2-carboxamidine
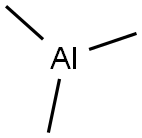
6-Trifluoromethyl-pyridine-2-carboxamidine Trimethyl aluminum (2.10 g, 29.11 mmol) was added dropwise to a vigorously stirred solution of NH4Cl (1.56 g, 29.11 mmol) in dry toluene (15 ml) at 0° C. The mixture was warmed room temperature and was stirred for 15 min. A solution of 6-trifluoromethyl-pyridine-2-carbonitrile (1.67 g, 9.703 mmol) in toluene (15 ml) was added dropwise. The reaction mixture was heated at 80° C. for 18 h. After cooling, the mixture was transferred to a vigorously stirred and cooled (0° C.) slurry of silica (20.0 g) in chloroform (150 ml) and was stirred for 10 min. The mixture was filtered and the filter cake was washed with MeOH (*3). The filtrate was concentrated in vacuo. The residue was dissolved in 1M HCl (150 ml) and Et2O (70 ml). The organic phase was separated and discarded. The aqueous phase was basified with saturated aqueous NaOH and extracted with chloroform (*2). The combined organic extracts were dried (Na2SO4) and concentrated in vacuo to give the title compound (980 mg, 53%). The compound could not be detected by HPLCMS, therefore structure was confirmed by NMR.
References:
US2012/202806,2012,A1
887583-52-0
82 suppliers
inquiry

790195-74-3
3 suppliers
inquiry
189278-27-1
256 suppliers
$8.00/1g

790195-74-3
3 suppliers
inquiry