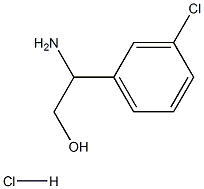
Benzeneethanol, β-aMino-3-chloro-, hydrochloride (1:1), (βS)- synthesis
- Product Name:Benzeneethanol, β-aMino-3-chloro-, hydrochloride (1:1), (βS)-
- CAS Number:620616-08-2
- Molecular formula:C8H11Cl2NO
- Molecular Weight:208.085
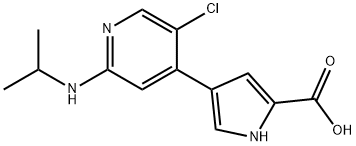
869886-90-8
10 suppliers
inquiry

620616-08-2
36 suppliers
$76.00/100mg
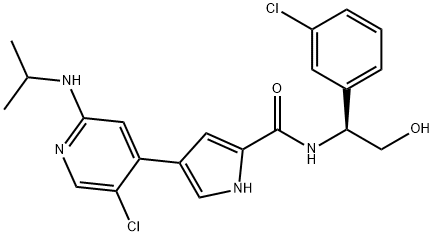
869886-67-9
143 suppliers
inquiry
Yield:-
Reaction Conditions:
with benzotriazol-1-ol;N-[3-(N,N-dimethylamino)-propyl]-N'-ethyl-carbodiimide hydrochloride;N-ethyl-N,N-diisopropylamine in DMF (N,N-dimethyl-formamide) at 20; for 16 h;Product distribution / selectivity;
Steps:
2
4-(5-chloro-2-isopropylaminopyridin-4-yl)-lH-pyrrole-2-carboxylic acid [1-(3- chlorophenyl)-2-hydroxyethyl] amide (1-9): 4-Bromo-2-chloro-5-N- isopropylpyridin-2-amine N-oxide (25 mg, 0.094 mmol, 1.0 equivalent) and 4- (4,4,5,5-tetraniethyl-[1 ,3,2]dioxaborolan-2-yl)-1 -(2,4,6-trimethylbenzensulfonyl)-lH- pyrrole-2-carboxylic acid methyl ester (39 mg, 0.094 mmol, 1.0 equivalent) were dissolved in benzene (5 mL) then aqueous 2M Na2C03 (1 mL) and Pd (PPh3)4 mg, 0.1 mmol, 0.2 equivalent) were added and the resulting suspension was heated at reflux at 80 °C for 16 hours. The reaction mixture was diluted in ethyl acetate, washed with water and dried over anhydrous sodium sulfate to afford 4-(5-chloro-2- isopropylamino-pyridin-4-yl)-1-(2,4,6-trimethyl-benzenesulfonyl)-1H pyrrole-2- carboxylic acid methyl ester N-oxide (Rt(min) 6.859. MS FIA: 492.0 ES+) which was then treated with a 2 M solution of PC13 in dichloromethane (1 mL) at room temperature. After 10 minutes, the solvent was removed under a stream of nitrogen and the crude oil was dissolved in methanol (1 mL) and aqueous 1 M NaOH (1 mL). The resulting mixture was heated at reflux for 16 hours then the crude solution was acidified using aqueous 1 M HCI and the solvent was removed. The resulting 4-(5- chloro-2-isopropylainino-pyridin-4-yl)-lH-pyrrole-2-carboxylic acid (Rt(min) 3.527. MS FIA: 279.4 ES+; 278.2 Es-) was suspended in DMF (3 mL) together with EDCI (36 mg, 0.19 mmol, 2 equivalents), HOBt (26 mg, 0.19 mmol, 2 equivalents), (S)-3- chlorophenylglycinol HCI salt (59 mg, 0.28 mmol, 3 equivalents) and DIEA (0.12 mL, 0.75 mmol, 8 equivalents). The resulting mixture was stirred at room temperature for 16 hours. The reaction mixture was diluted in ethyl acetate, washed with water and dried over sodium sulfate. After removing the solvent under reduced pressure, the crude product was purified by reversed phase HPLC (acetonitrile/water/TFA 1 %) to afford the title compound as a white solid (4.8 mg, 8.1%).
References:
WO2005/113541,2005,A1 Location in patent:Paragraph 0090