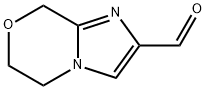
8H-Imidazo[2,1-c][1,4]oxazine-2-carboxaldehyde, 5,6-dihydro- (9CI) synthesis
- Product Name:8H-Imidazo[2,1-c][1,4]oxazine-2-carboxaldehyde, 5,6-dihydro- (9CI)
- CAS Number:623564-42-1
- Molecular formula:C7H8N2O2
- Molecular Weight:152.15
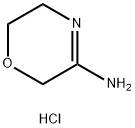
623564-41-0
![8H-Imidazo[2,1-c][1,4]oxazine-2-carboxaldehyde, 5,6-dihydro- (9CI)](/CAS/GIF/623564-42-1.gif)
623564-42-1
![8H-Imidazo[2,1-c][1,4]oxazine-3-carboxaldehyde, 5,6-dihydro- (9CI)](/CAS/GIF/623564-43-2.gif)
623564-43-2
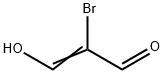
Step 5: Synthesis of 5,6-dihydro-8H-imidazo[2,1-c][1,4]oxazine-2-carboxaldehyde (9) and 6,8-dihydro-5H-imidazo[2,1-c][1,4]oxazine-3-carboxaldehyde; a mixture of 2-bromo-3-hydroxyacrylaldehyde (4.1 g), p-toluenesulfonic acid monohydrate (52 mg) and 2-propanol (5.2 mL) in cyclohexane (42 mL ) in a mixture was subjected to azeotropic dehydration until the vapor temperature rose to 80 °C. The reaction mixture was concentrated under reduced pressure and the residue was dissolved in anhydrous ethanol (50 mL). To this solution was added a mixture of a solution of 3-iminomorpholine hydrochloride (3.4 g) in anhydrous ethanol (200 mL) and a methanol solution of 28% sodium methanol (4.8 g) at room temperature. The reaction mixture was stirred at room temperature for 2 hours before the solvent was removed under vacuum. The residue was dissolved in chloroform (125 mL), triethylamine (3.5 mL) was added and the reaction mixture was subsequently heated to reflux for 2 hours. After cooling to room temperature, the reaction mixture was concentrated under reduced pressure. The residue was dissolved in dichloromethane (300 mL) and washed with 50% aqueous K2CO3 (2 x 100 mL). The organic layer was dried over anhydrous magnesium sulfate and filtered, and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography using CHCl3-acetone (4:1) as eluent to afford the target product 5,6-dihydro-8H-imidazo[2,1-c][1,4]oxazine-2-carboxaldehyde (light orange solid, 1.4 g, 36.3% yield) and another regional isomer 6,8-dihydro-5H-imidazo[2,1-c][1,4]oxazine-3 -formaldehyde (light orange solid, 609 mg, 16.1% yield). 1H NMR (CDCl3) δ of the target product: 4.08-4.15 (m, 4H), 4.88 (s, 2H), 7.58 (s, 1H), 9.85 (s, 1H). 1H NMR (CDCl3) δ of the regional isomer: 4.06 (t, 2H, J=5.2Hz), 4.40 (t, 2H, J=5.2Hz), 4.90 (s, 2H), 7.75 (s, 1H), 9.72 (s, 1H).
![8H-Imidazo[2,1-c][1,4]oxazine-2-carboxylic acid, 5,6-dihydro-, ethyl ester](/CAS/20210305/GIF/1096418-58-4.gif)
1096418-58-4
2 suppliers
inquiry
![8H-Imidazo[2,1-c][1,4]oxazine-2-carboxaldehyde, 5,6-dihydro- (9CI)](/CAS/GIF/623564-42-1.gif)
623564-42-1
39 suppliers
$130.00/50mg
Yield:623564-42-1 41%
Reaction Conditions:
Stage #1: C9H12N2O3with diisobutylaluminium hydride in tetrahydrofuran at -78 - 40; for 3 h;
Stage #2: with water;ammonium chloride in tetrahydrofuran;Product distribution / selectivity;
Steps:
9
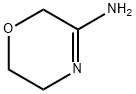
Example 9Alternative Preparation of 5,6-Dihydro-8H-imidazo[2,1-c][1,4]oxazine-2-carbaldehyde (1); To a stirred solution of morpholin-3-ylideneamine hydrochloride salt (1.3 g, 10 millimolar (mmol)) in DME (50 mL) at room temperature was added an excess of anhydrous K2CO3 and the mixture stirred for 10 minutes. Then ethyl-bromopyruvate (10) (2.94 g, 15 mmol) was added and the mixture stirred at room temperature for 4 hours, then refluxed for 16 hours. Examination of TLC by UV visualization using 1:1:0.05 ethylacetate:hexane:methanol showed only one product. The reaction mixture was concentrated and extracted with chloroform. The chloroform extract was dried over anhydrous Na2SO4, filtered, and concentrated. The crude product was purified by SiO2 column chromatography using 1:1:0.05 ethylacetate:hexane:methanol as eluent, yielding 980 mg of a pale yellow liquid (50% yield).The intermediate ester (11) (600 mg, 3.06 mmol) was dissolved in anhydrous THF (50 mL) and cooled to -78° C. To the stirred reaction mixture, DIBAL (1 molar (M) solution, 3.5 mL) was slowly added. The reaction mixture was stirred for 2 hours while the temperature was slowly elevated to -40° C. and then stirred for 1 hour at 40° C. The reaction mixture was then quenched with a saturated NH4Cl solution and extracted with chloroform. The chloroform extract was washed once with a saturated brine solution. The organic layer was dried over anhydrous Na2SO4 and filtered. It was concentrated and purified by SiO2 column chromatography, eluting with ethylaceate:hexane (4:1), yielding 250 mg (41%) of the product Compound 1 having the same 1H-NMR spectrum as above.
References:
US2009/18332,2009,A1 Location in patent:Page/Page column 14

623564-41-0
4 suppliers
inquiry
![8H-Imidazo[2,1-c][1,4]oxazine-2-carboxaldehyde, 5,6-dihydro- (9CI)](/CAS/GIF/623564-42-1.gif)
623564-42-1
39 suppliers
$130.00/50mg
![8H-Imidazo[2,1-c][1,4]oxazine-3-carboxaldehyde, 5,6-dihydro- (9CI)](/CAS/GIF/623564-43-2.gif)
623564-43-2
28 suppliers
inquiry

623564-41-0
4 suppliers
inquiry
19263-02-6
1 suppliers
inquiry
![8H-Imidazo[2,1-c][1,4]oxazine-2-carboxaldehyde, 5,6-dihydro- (9CI)](/CAS/GIF/623564-42-1.gif)
623564-42-1
39 suppliers
$130.00/50mg
![8H-Imidazo[2,1-c][1,4]oxazine-3-carboxaldehyde, 5,6-dihydro- (9CI)](/CAS/GIF/623564-43-2.gif)
623564-43-2
28 suppliers
inquiry

623564-41-0
4 suppliers
inquiry

155272-73-4
7 suppliers
inquiry
![8H-Imidazo[2,1-c][1,4]oxazine-2-carboxaldehyde, 5,6-dihydro- (9CI)](/CAS/GIF/623564-42-1.gif)
623564-42-1
39 suppliers
$130.00/50mg
![8H-Imidazo[2,1-c][1,4]oxazine-3-carboxaldehyde, 5,6-dihydro- (9CI)](/CAS/GIF/623564-43-2.gif)
623564-43-2
28 suppliers
inquiry
747408-16-8
9 suppliers
inquiry

155272-73-4
7 suppliers
inquiry
![8H-Imidazo[2,1-c][1,4]oxazine-2-carboxaldehyde, 5,6-dihydro- (9CI)](/CAS/GIF/623564-42-1.gif)
623564-42-1
39 suppliers
$130.00/50mg
![8H-Imidazo[2,1-c][1,4]oxazine-3-carboxaldehyde, 5,6-dihydro- (9CI)](/CAS/GIF/623564-43-2.gif)
623564-43-2
28 suppliers
inquiry