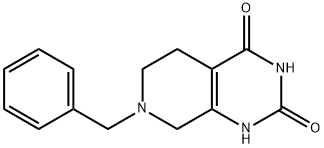
7-BENZYL-5,6,7,8-TETRAHYDROPYRIDO[3,4-D]PYRIMIDINE-2,4(1H,3H)-DIONE synthesis
- Product Name:7-BENZYL-5,6,7,8-TETRAHYDROPYRIDO[3,4-D]PYRIMIDINE-2,4(1H,3H)-DIONE
- CAS Number:62459-02-3
- Molecular formula:C14H15N3O2
- Molecular Weight:257.29
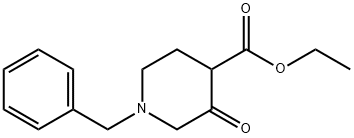
39514-19-7
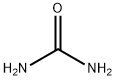
57-13-6
![7-BENZYL-5,6,7,8-TETRAHYDROPYRIDO[3,4-D]PYRIMIDINE-2,4(1H,3H)-DIONE](/CAS/GIF/62459-02-3.gif)
62459-02-3
To a solution of ethyl 1-benzyl-3-oxa-4-piperidinecarboxylate (12.0 g, 40.4 mmol) in methanol (200 ml) at 0 °C was added sequentially urea (5.1 g, 84.8 mmol) and sodium methanolate (12.3 g, 228 mmol). The reaction mixture was stirred at 60°C for 96 hours under nitrogen protection. After completion of the reaction, the mixture was cooled to room temperature and concentrated under reduced pressure to remove the solvent. The residue was purified by silica gel column chromatography (eluent: dichloromethane/methanol=10:1, v/v) to afford 7-benzyl-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidine-2,4(1H,3H)-dione (6.3 g, 61% yield). lRMS (M+H+) m/z: calculated value 258.29; measured value 258.30.
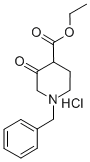
52763-21-0
242 suppliers
$6.00/1g

57-13-6
798 suppliers
$5.00/5g
![7-BENZYL-5,6,7,8-TETRAHYDROPYRIDO[3,4-D]PYRIMIDINE-2,4(1H,3H)-DIONE](/CAS/GIF/62459-02-3.gif)
62459-02-3
126 suppliers
$56.00/25 mg
Yield: 83.2%
Reaction Conditions:
with sodium in ethanol at 75; for 36 h;
Steps:
A Step A: 7-benzyl-6.8-dihydro-5H-pyrido[3.4-dlpyrimidine-2.4-diol:
To EtOH (600 mL) was added Na (5.56 g, 241 mmol, 5.73 mL, 2.40 eq) in portions. The reaction mixture was stirred for 1 hour. To the mixture was added ethyl l-benzyl-3-oxo-piperidine-4-carboxylate (30.0 g, 100 mmol, 1.00 eq, HC1) and urea (14.5 g, 242 mmol, 13.0 mL, 2.40 eq). The reaction mixture was stirred at 75 °C for 36 hours, and then the solvent was removed under vacuum. The residue was dissolved in water (50 mL) and acidified with HC1 (120 mL, 2M). A white solid precipitated from the solution and was collected by filtration. The filter cake was dried under vacuum to provide 7-benzyl-6,8-dihydro-5H-pyrido[3,4-d]pyrimidine-2,4-diol (22.0 g, 83.8 mmol, 83.2 % yield, 98 % purity) as a white solid.1HNMR (400 MHz, DMSO-de) δ = 10.97 (br s, 1H), 10.66 (br s, 1H), 7.55 - 6.95 (m, 5H), 3.81 - 3.50 (m, 2H), 3.26 - 2.91 (m, 2H), 2.77 - 2.58 (m, 2H), 2.34 - 2.09 (m, 2H).
References:
MIRATI THERAPEUTICS, INC.;ARRAY BIOPHARMA, INC.;FISCHER, John, P.;FELL, Jay, Bradford;BLAKE, James, F.;HINKLIN, Ronald, Jay;MEJIA, Macedonio, J.;HICKEN, Erik, James;CHICARELLI, Mark, Joseph;GAUDINO, John, J.;VIGERS, Guy, P.A.;BURGESS, Laurence, E.;MARX, Matthew, Arnold;CHRISTENSEN, James, Gail;LEE, Matthew, Randolf;SAVECHENKOV, Pavel;ZECCA, Henry, J. WO2017/201161, 2017, A1 Location in patent:Paragraph 0221; 0267

39514-19-7
136 suppliers
$17.00/1g

57-13-6
798 suppliers
$5.00/5g
![7-BENZYL-5,6,7,8-TETRAHYDROPYRIDO[3,4-D]PYRIMIDINE-2,4(1H,3H)-DIONE](/CAS/GIF/62459-02-3.gif)
62459-02-3
126 suppliers
$56.00/25 mg