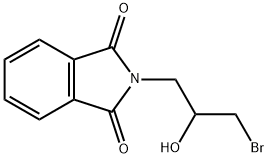
1H-Isoindole-1,3(2H)-dione, 2-(3-bromo-2-hydroxypropyl)- synthesis
- Product Name:1H-Isoindole-1,3(2H)-dione, 2-(3-bromo-2-hydroxypropyl)-
- CAS Number:6284-27-1
- Molecular formula:C11H10BrNO3
- Molecular Weight:284.11
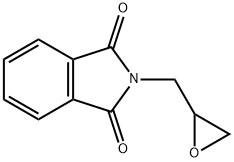
5455-98-1
165 suppliers
$25.00/5g

6284-27-1
1 suppliers
inquiry
Yield:6284-27-1 98%
Reaction Conditions:
with water;hydrogen bromide in chloroform at 0; for 0.5 h;regioselective reaction;
Steps:
Preparation of aminoalcohols 3a-e via formal addition of HX acid. (General Procedure A)
General procedure: To a solution of N-(2,3-epoxypropyl)phthalimide (2) (1.0 equiv.), in chloroform (10 mL) cooled at 0°C, a concentrated solution of hydrohalic acid (2.0 equiv.) was added dropwise. The mixture was allowed to react during 0.5-6 h (Table 1) and, therefore it was washed with saturated aqueous NaCl and extracted twice with dichloromethane. Organic phases were dried over anhydrous sodium sulfate, filtered and after removal of the organic solvents in vacuo pure compounds were obtained without needing of further purification.
References:
Pace, Vittorio;Holzer, Wolfgang [Tetrahedron Letters,2012,vol. 53,# 38,p. 5106 - 5109] Location in patent:supporting information; experimental part
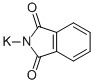
1074-82-4
468 suppliers
$5.00/25g

6284-27-1
1 suppliers
inquiry