
N-[2-(2-AMINO-4-CHLOROPHENOXY)ETHYL]-N,N-DIMETHYLAMINE synthesis
- Product Name:N-[2-(2-AMINO-4-CHLOROPHENOXY)ETHYL]-N,N-DIMETHYLAMINE
- CAS Number:631862-75-4
- Molecular formula:C10H15ClN2O
- Molecular Weight:214.69
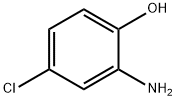
95-85-2
404 suppliers
$13.00/25g
![N-[2-(2-AMINO-4-CHLOROPHENOXY)ETHYL]-N,N-DIMETHYLAMINE](/StructureFile/ChemBookStructure21/GIF/CB5800885.gif)
631862-75-4
25 suppliers
$60.00/5mg
Yield:631862-75-4 72%
Reaction Conditions:
with potassium carbonate in (2-Chloro-ethyl)-dimethyl-amine.hydrochloride;acetone;
Steps:
2.1 5-Chloro-2-(2-dimethylamino-ethoxy)-phenylamine
Step 1 5-Chloro-2-(2-dimethylamino-ethoxy)-phenylamine To a suspension of 2-Amino-4-chloro-phenol (1.43 g, 10 mmoles, 1 eq) and potassium carbonate (6.9 g, 50 mmoles, 5 eq) in acetone (25 ml) was added in one portion (2-Chloro-ethyl)-dimethyl-amine.hydrochloride (2.16 g, 15 mmoles, 1.5 eq). The reaction mixture was refluxed under Nitrogen for 6 hours at which time the suspension was filtered and the solid washed with acetone. The combined filtrate was concentrated under vacuum and purified by flash chromatography (EtOAc/MeOH/NEt3: 87/9/3) to afford a beige solid (1.6 g, yield=72%). 1H NMR (400 MHz, DMSO-d6) δ 6.78 (d, 1H, J=8.6); 6.63 (d, 1H, J=2.5); 6.47 (dd, 1H, J=8.6, 2.5); 5.03 (s, 2H, broad); 3.88 (t, 2H, J=5.8); 2.60 (t, 2H, J=5.8); 2.21 (s, 6H). LCMS: method A, RT=0.5 min, [MH+=215].
References:
US2004/14765,2004,A1
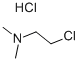
4584-46-7
0 suppliers
$21.69/100g

95-85-2
404 suppliers
$13.00/25g
![N-[2-(2-AMINO-4-CHLOROPHENOXY)ETHYL]-N,N-DIMETHYLAMINE](/StructureFile/ChemBookStructure21/GIF/CB5800885.gif)
631862-75-4
25 suppliers
$60.00/5mg
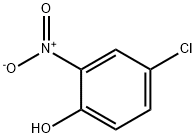
89-64-5
13 suppliers
$31.80/25g
![N-[2-(2-AMINO-4-CHLOROPHENOXY)ETHYL]-N,N-DIMETHYLAMINE](/StructureFile/ChemBookStructure21/GIF/CB5800885.gif)
631862-75-4
25 suppliers
$60.00/5mg