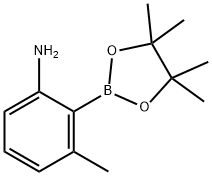
3-METHYL-2-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL) BENZENAMINE synthesis
- Product Name:3-METHYL-2-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL) BENZENAMINE
- CAS Number:631909-35-8
- Molecular formula:C13H20BNO2
- Molecular Weight:233.11
54879-20-8
166 suppliers
$6.00/1g

73183-34-3
587 suppliers
$6.00/5g

631909-35-8
17 suppliers
inquiry
Yield:631909-35-8 65%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate in 1,4-dioxane at 90; for 18 h;Inert atmosphere;Sealed tube;
Steps:
Intermediate T: 3-Methyl-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)aniline
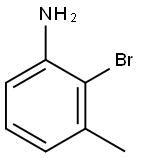
To a solution of 2-bromo-3-methylaniline (0.34 ml_, 2.7 mmol) in 1 ,4-dioxane (12 ml_) was added potassium acetate (527 mg, 5.37 mmol), bis(pinacolato)diboron (1365 mg, 5.37 mmol) and the solution was degassed for 10 min. [1,1'- (0296) Bis(diphenylphosphino)ferrocene]dichloropalladium(ll), complex with dichloromethane (219 mg, 0.269 mmol) were added and the suspension was heated to 90 °C for 18h in a closed vessel. The reaction mixture was allowed to cool, diluted with EtOAc then washed with brine. The organic layer was dried over MgSCU, and concentrated in vacuo. The residue was purified by chromatography (S1O2, 0-20% EtOAc in PE) and the title compound was isolated as a white solid (742 mg, 65 %). 1H NMR dH (500 MHz, CDCh) 7.04 (t, J = 7.7 Hz, 1 H), 6.49 (m, 2H), 4.20 (s, 2H), 2.44 (s, 3H), 1.34 (s, 12H). LCMS (Method A): 4.15 min (234.2, MH+).
References:
WO2019/141972,2019,A1 Location in patent:Page/Page column 39