(E)-2-(2-fluorostyryl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane synthesis
- Product Name:(E)-2-(2-fluorostyryl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
- CAS Number:633327-38-5
- Molecular formula:C14H18BFO2
- Molecular Weight:248.1
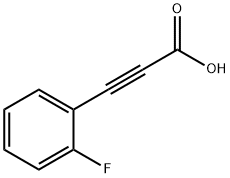
766-49-4
164 suppliers
$10.00/1g
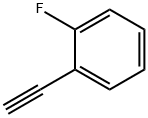
25015-63-8
225 suppliers
$22.39/5G

633327-38-5
14 suppliers
inquiry
Yield:633327-38-5 99%
Reaction Conditions:
with (1,3-bis(2,6-di-iso-propylphenyl)-4,5-dihydroimidazol-2-ylidene) copper chloride;LiNPtBu3 in benzene at 20; for 13 h;Glovebox;Inert atmosphere;stereoselective reaction;
Steps:
III. Procedure for the Cu(I)-Catalyzed Alkyne Hydroboration Reaction 1. Cu (I) catalyzed hydroboration of terminal alkyne (Table 2)
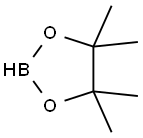
General procedure: In an N2 charged glove box with oxygen and water levels ≤0.1 ppm, to an oven-dried screwed vial were added SIPrCuCl (c8) (4.9 mg, 5 mol%, 0.01 mmol), tri(t-Butyl)phosphoranimide (1b) (2.2 mg, 5 mol%, 0.01 mmol), Pinacolborane (1c) (28 mg, 0.22 mmol, 1.1 eq.), terminal alkyne (1) (0.2 mmol, 1 eq.) and benzene (2 mL, 0.1 M). The formed reaction mixture was kept stirring for 13-15 hours in glove box. Then the dark brown reaction mixture was taken out from glove box the solvent was removed by rotary evaporator. The solvent mixture (Hexane:Ethyl acetate) was added into the reaction residue as eluent then kept stirring for about 30 mins under room temperature until the precipitate was formed. The mixture was further filtered through a short pad of silica gel to get colorless filtrate. Organic solvents were removed under reduced pressure and desired product was obtained after dryness under vacuum without further purification.
References:
Bai, Tao;Yang, Yanhui;Han, Chao [Tetrahedron Letters,2017,vol. 58,# 15,p. 1523 - 1527] Location in patent:supporting information
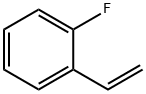
394-46-7
152 suppliers
$9.00/100mg

25015-63-8
225 suppliers
$22.39/5G

633327-38-5
14 suppliers
inquiry
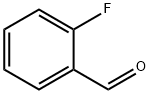
446-52-6
441 suppliers
$6.00/25g
![Bis[(pinacolato)boryl]Methane](/CAS/GIF/78782-17-9.gif)
78782-17-9
104 suppliers
$22.00/1g

633327-38-5
14 suppliers
inquiry