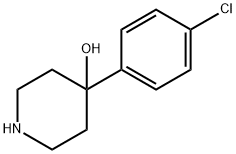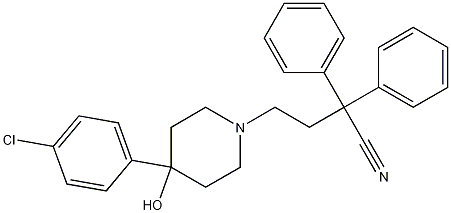
4-(4-Chlorophenyl)-4-hydroxy-a,a-diphenyl-1-piperidinebutanenitrile synthesis
- Product Name:4-(4-Chlorophenyl)-4-hydroxy-a,a-diphenyl-1-piperidinebutanenitrile
- CAS Number:63959-33-1
- Molecular formula:C27H27ClN2O
- Molecular Weight:430.97
Yield:63959-33-1 77%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in acetonitrile; for 40 h;Inert atmosphere;Reflux;
Steps:
4.2.17 4-(4-(4-Chlorophenyl)-4-hydroxypiperidin-1-yl)-2,2-diphenylbutanenitrile (25a)
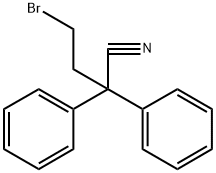
Under inert conditions, 4-(4-Chlorophenyl)-4-hydroxypiperidine (27a) (1.78g, 8.41mmol, 1.0equiv.) was suspended in 28mL MeCN and DIPEA (4.30mL, 23.2mmol, 3.0equiv.) was added. 4-Bromo-2,2-diphenylbutyronitrile (26) (2.52g, 8.41mmol, 1.0equiv.) was then added and the reaction mixture was stirred at reflux for 40h. After concentration under reduced pressure, the crude residue was purified by flash chromatography (eluent DCM/MeOH=98/2 to 9/1), yielding 2.78g (77%) of diphenylbutanenitrile ( 25a) as a white solid. (0076) Rf (DCM/MeOH=9/1)=0.38; 1H NMR (400MHz, CDCl3) δ (ppm)=7.28-7.46 (14H, m, Ar-H), 2.40-2.90 (8H, m, H-2, H-3 and H-4), 2.00-2.21 (2H, m, H-5a), 1.71 (2H, d, H-5b, J=12.9Hz), 1.57 (1H, bs, OH).
References:
Boudhar, Aicha;Ng, Xiao Wei;Loh, Chiew Yee;Chia, Wan Ni;Tan, Zhi Ming;Nosten, Francois;Dymock, Brian W.;Tan, Kevin S.W. [European Journal of Medicinal Chemistry,2016,vol. 119,p. 231 - 249]