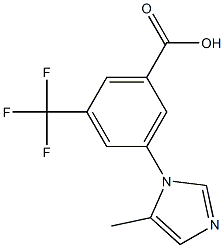
3-(5-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)benzoic acid synthesis
- Product Name:3-(5-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)benzoic acid
- CAS Number:641571-18-8
- Molecular formula:C12H9F3N2O2
- Molecular Weight:270.2073
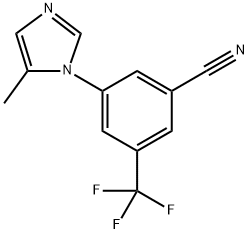
641571-17-7
0 suppliers
inquiry

641571-18-8
7 suppliers
inquiry
Yield:641571-18-8 47.6%
Reaction Conditions:
with water;sodium hydroxide in 1,4-dioxane at 95;Inert atmosphere;
Steps:
6.13 Step 13: Synthesis of 3-( -methyl-lH-imidazol-l-yl)-5-(trifluoromethyl)benzonitrile (13C)
To a stirred solution of 3-(5-methyl-lH-imidazol-l-yl)-5-(trifluoromethyl)benzonitrile (13C) (12.6 g, 50.2 mmol, 1.0 equiv) in 1,4-dioxane (252.0 mL, 20.0 vol. equiv) was added a solution of sodium hydroxide (10.0 g, 251.0 mmol, 5.0 equiv) in water (315.0 mL, 25.0 vol. equiv) at RT. The resulting mixture was stirred for 15 h at 95 °C. The completion of reaction was monitored by TLC. The reaction mixture was concentrated under vacuum and the residue was dissolved in water, adjust pH acidic with 2N HC1 and extracted with ethyl acetate (3 x 150.0 mL). The combined organic extract was washed with brine (150.0 mL), dried over sodium sulphate and concentrated under vacuum to afford titled compound (13) (6.45 g, 47.6%) as an off white solid. NMR (300 MHz, OMSO-d6) δ 8.28-8.24 (t, 3H), 8.05-7.99 (d, 2H), 7.59 (s, 1H), 2.49-2.37 (m, 3H). MS (ES+): 271 (M+l); MS (ES-): 269.3 (M-l).
References:
WO2018/200981,2018,A1 Location in patent:Paragraph 0209; 0242
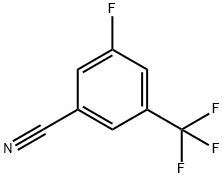
149793-69-1
192 suppliers
$10.00/2g

641571-18-8
7 suppliers
inquiry