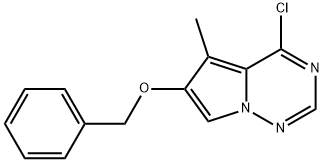
6-Benzyloxy-4-chloro-5-methylpyrrolo[2,1-f][1,2,4]triazine synthesis
- Product Name:6-Benzyloxy-4-chloro-5-methylpyrrolo[2,1-f][1,2,4]triazine
- CAS Number:649736-27-6
- Molecular formula:C14H12ClN3O
- Molecular Weight:273.72
![Pyrrolo[2,1-f][1,2,4]triazin-4(1H)-one, 5-methyl-6-(phenylmethoxy)-](/CAS/GIF/649736-26-5.gif)
649736-26-5
16 suppliers
$668.00/100mg
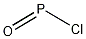
21295-50-1
2 suppliers
inquiry
![6-Benzyloxy-4-chloro-5-methylpyrrolo[2,1-f][1,2,4]triazine](/CAS/GIF/649736-27-6.gif)
649736-27-6
11 suppliers
inquiry
Yield:649736-27-6 93%
Reaction Conditions:
with 1,1-diisopropylethylamine in toluene at 85; for 5 h;
Steps:
1.G G. 6-benzyloxy-4-chloro-5-methylpyrrolo[2,1-f][1,2,4]triazine
A mixture of compound F of this example (10 g, 39.2 mmol), phosphorus oxychloride (4.4 mL, 47.1 mmol) and diisopropylethyl amine (5.5 mL, 31.4 mmol) in toluene (150 ml) was stirred at 85 C for 2 h and then more phosphorus oxychloride (1.1 mL, 11.8 mmol) was added. After 2 h, additional phosphorus oxychloride (1.1 mL, 11.8 mmol) was added. The reaction mixture was continuously stirred at 85 C for lh and then concentrated. The residue was dissolved in dichloromethane, washed with cold sodium bicarbonate solution, dried, and concentrated in vacuo. The crude material was purified by chromatography on silica gel eluting with dichloromethane to provide Compound G (9.9 g, 93 %) as a yellow solid
References:
WO2004/9542,2004,A2 Location in patent:Page 29
![Pyrrolo[2,1-f][1,2,4]triazin-4(1H)-one, 5-methyl-6-(phenylmethoxy)-](/CAS/GIF/649736-26-5.gif)
649736-26-5
16 suppliers
$668.00/100mg
![6-Benzyloxy-4-chloro-5-methylpyrrolo[2,1-f][1,2,4]triazine](/CAS/GIF/649736-27-6.gif)
649736-27-6
11 suppliers
inquiry

310444-84-9
2 suppliers
inquiry
![6-Benzyloxy-4-chloro-5-methylpyrrolo[2,1-f][1,2,4]triazine](/CAS/GIF/649736-27-6.gif)
649736-27-6
11 suppliers
inquiry

886585-68-8
1 suppliers
inquiry
![6-Benzyloxy-4-chloro-5-methylpyrrolo[2,1-f][1,2,4]triazine](/CAS/GIF/649736-27-6.gif)
649736-27-6
11 suppliers
inquiry