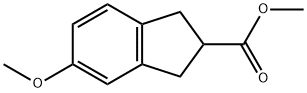
Methyl 5-Methoxy-2,3-dihydro-1H-indene-2-carboxylate synthesis
- Product Name:Methyl 5-Methoxy-2,3-dihydro-1H-indene-2-carboxylate
- CAS Number:65844-46-4
- Molecular formula:C12H14O3
- Molecular Weight:206.24
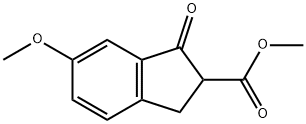
22955-78-8
9 suppliers
inquiry

64-19-7
1628 suppliers
$10.00/25ML

65844-46-4
22 suppliers
$45.00/10mg
Yield:65844-46-4 65%
Reaction Conditions:
with perchloric acid;5%-palladium/activated carbon at 20; under 30402 Torr; for 12 h;Hydrogen atmosphere;
Steps:
Intermediate 28; 5-Methoxy-indan-2-carboxylic acid methyl ester.
Combine in a Parr shaker 6-Methoxy-1-oxo-indan-2-carboxylic acid methyl ester (3. 14g, 14. 25 mmol), added acetic acid (150 ml), perchloric acid (0.8 mL) and 5% Pd-C (0.14 mmol). After the reaction has been on the parr shaker under 40 atm of H2 pressure at room temperature for 12 hours, filter the reaction mixture through a pad of Celite using ethyl acetate eluent. Then add the filtrate to a separatory funnel and wash with water then brine, and dry the organic layer over NA2S04. After concentrating under reduced pressure, flash Chromatograph using 8 : 1 Hexanes : Ethyl acetate to afford 1. 9g, 9. 21mmol (65% yield) of the title compound as a clear oil : 1HNMR (500 MHz, CDCl3) ; 3. 2-3. 4 (5H, m), 3.7 (3H, s), 3.8 (3H, s), 6.7-6.8 (2H, m), 7.1-7.2 (1H, m) ; TLC 1:1 Hexanes : Ethyl acetate RF: =0.6.
References:
WO2004/80968,2004,A1 Location in patent:Page 58

22955-78-8
9 suppliers
inquiry

65844-46-4
22 suppliers
$45.00/10mg

58461-21-5
1 suppliers
inquiry

65844-46-4
22 suppliers
$45.00/10mg
13623-25-1
302 suppliers
$6.00/1g

65844-46-4
22 suppliers
$45.00/10mg