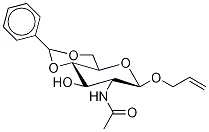
.beta.-D-Glucopyranoside, 2-propenyl 2-(acetylamino)-2-deoxy-4,6-O-(phenylmethylene)- synthesis
- Product Name:.beta.-D-Glucopyranoside, 2-propenyl 2-(acetylamino)-2-deoxy-4,6-O-(phenylmethylene)-
- CAS Number:65947-37-7
- Molecular formula:C18H23NO6
- Molecular Weight:349.38
1125-88-8
364 suppliers
$6.00/25g

65947-37-7
19 suppliers
$94.50/5mg
Yield:65947-37-7 85%
Reaction Conditions:
Stage #1: allyl 2-acetamido-3,4,6-tri-O-acetyl-2-deoxy-β-D-glucopyranosidewith methanol;sodium methylate
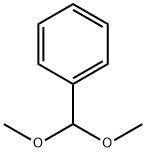
Stage #2: benzaldehyde dimethyl acetalwith (1S)-10-camphorsulfonic acid in N,N-dimethyl-formamide;
Steps:
1.3.J
(J) (data of glycosyl acceptor) allyl-O-3,6-di-O-benzyl-2-deoxy-2-phthalimide-β-D-glucopyranoside (12); Amino-protected glucopyranoside (12) was synthesized according to the synthetic scheme shown below. [Show Image] 1H NMR δ(CDCl3) 3.63(m, 1H, H-5), 3.76-3.85(m, 3H, H-4, H-6a and H-6b), 3.97(dd, 1H, J=13.1Hz, J=6.1Hz, CHH'CH=CH2), 4.15-4.26(m, 3H, H-2, H-3 and CHH'CH=CH2), 4.52 and 4.73(ABq, 2H, J=12.2Hz, PhCH2), 4.58 and 4.64(ABq, 2H, J=11.9Hz, PhCH2), 4.99(dd, 1H, J=1.3Hz, Jtrans=10.3Hz, CH=CHcisHtrans), 5.07(dd, 1H, J=1.3Hz, Jcis=17.2Hz, CH=CHcisHtrans), 5.17(d, 1H, J1, 2=8.1Hz, H-1), 5.61-5.70(m, 1H, CH=CH2), 6.93-7.67(m, 14H, Ar-H) 13C NMR δ(CDCl3) 55.3(C-2), 69.7(C-C=C), 70.7(C-6), 73.5(C-5), 73.8 and 74.3(Ph-C), 74.5(C-4), 78.7(C-3), 97.4(C-1), 117.3(C-C=C), 127.4-128.5(m, Ar-C), 133.6(C-C=C), 137.6 and 138.2(C=O) HR-FAB MS[M+H]+Calculated for C31H32NO7 530.218, Found 530.215 t.l.c; Rf = 0.72 (toluene/ethyl acetate = 1:1)
References:
EP1698634,2006,A1 Location in patent:Page/Page column 15-16