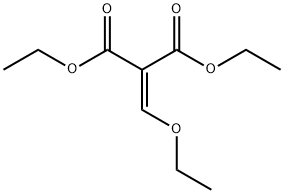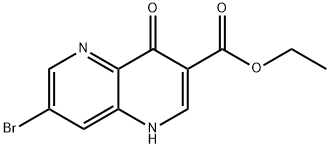
7-Bromo-1,5-naphthyridine-4-oxo-3-carboxylic acid ethyl ester synthesis
- Product Name:7-Bromo-1,5-naphthyridine-4-oxo-3-carboxylic acid ethyl ester
- CAS Number:66093-18-3
- Molecular formula:C11H9BrN2O3
- Molecular Weight:297.1
![Propanedioic acid, 2-[[(5-bromo-3-pyridinyl)amino]methylene]-, 1,3-diethyl ester](/CAS/20210305/GIF/64436-94-8.gif)
64436-94-8
0 suppliers
inquiry

66093-18-3
8 suppliers
inquiry
Yield:-
Reaction Conditions:
in diphenylether; for 1 h;Reflux;
Steps:
1.2 Ethyl 7-bromo-4-oxo-1,4-dihydro-1,5-naphthyridine-3-carboxylate
Step 2:
Ethyl 7-bromo-4-oxo-1,4-dihydro-1,5-naphthyridine-3-carboxylate
In a RBF fitted with a reflux condenser a mixture of diethyl 2-((5-bromopyridin-3-ylamino)methylene)malonate (1 g, 3 mmol) and 1-phenoxybenzene (10 mL, 63 mmol) was heated to reflux for 1 h.
The reaction mixture was allowed to cool to RT and CH3CN was added.
The resulting solids were filtered and dried to provide the desired product as a light brown solid.
References:
US2014/336182,2014,A1 Location in patent:Paragraph 0300
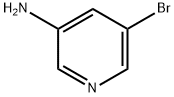
13535-01-8
390 suppliers
$3.00/1g

66093-18-3
8 suppliers
inquiry
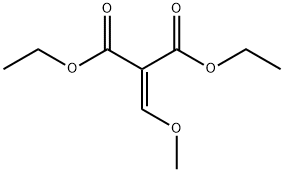
40131-09-7
4 suppliers
inquiry

66093-18-3
8 suppliers
inquiry